Division of Pediatric Glioma Research
Dr. David T.W. Jones
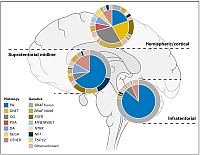
Approximation of the frequencies of different histological groupings of pediatric low-grade glioma and associated molecular genetic alterations, split by location within the brain. Adapted from Sturm D, Pfister SM and Jones DTW, J Clin Oncol 2017.
© dkfz.de
Gliomas are the most common form of brain tumor found in children, in total accounting for about half of all central nervous system tumors in 0-19 year olds. Two major categories are usually distinguished: low-grade and high-grade glioma.
Low-grade gliomas are a histologically and biologically varied collection of tumor entities, which are often defined by genetic alterations in the mitogen-activated protein kinase (MAPK) pathway and are typically considered as benign tumors due to their good overall survival rates. This survival, however, often comes at a significant cost to quality of life due to effects of treatment as well as the tumor itself. Multiple recurrences over several years are also not uncommon, creating a heavy burden on patients and their families. Our research aims to learn more about the background of these tumors and their unusual growth behaviour, as well as to identify treatments that are more tailored to individual tumor biology and therefore hopefully have fewer side effects.
High-grade gliomas, in contrast, carry an extremely dismal prognosis. These highly aggressive tumors, most commonly glioblastoma or diffuse intrinsic pontine glioma (DIPG), typically display a much greater degree of genomic instability than lower-grade lesions. Combined disruption of multiple cellular processes leads to rapidly dividing cancer cells that diffusely infiltrate the surrounding normal brain tissue, with an almost universally fatal outcome. Our research in this area aims at understanding the heterogeneity both within single tumors and between individuals, to identify the most important patterns of alterations in the cellular machinery. We then try to recapitulate these changes in model systems, in order to understand their contribution to a cell becoming cancerous and also to test new therapies in a rationally targeted manner.
All of the work being conducted in the lab is based on the application of cutting-edge genomic (next-generation DNA/RNA sequencing), epigenomic (DNA methylation, ChIPseq), and functional technologies (CRISPR/Cas9, somatic gene transfer models) to enhance our understanding of the biological underpinnings of pediatric brain tumors. A further important focus of the group is the translation of this research into practical applications for the benefit of patients. To this end, we are closely involved in the coordination of two international molecular diagnostic programs: the INFORM study for identification of possible drug targets in high-risk pediatric cancers; and the MNP2 study to investigate the value of molecular analysis as a tool for accurate classification of brain tumors.
More information on the Pediatric Neurooncology community in Heidelberg can be found here.

