Melanoma microRNA trafficking controls tumour primary niche formation



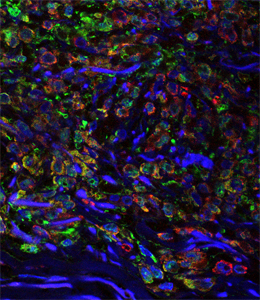
Melanoma tumour in situ. The melanoma cells have not yet left the epidermis; green: fibroplasts; red: melanosomes; blue: DNA in cell nuclei.
© dkfz.de
Melanoma originates in the epidermis and becomes metastatic after invasion into the dermis. Prior interactions between melanoma cells and dermis are poorly studied. Here, we show that melanoma cells directly affect the formation of the dermal tumour niche by microRNA trafficking before invasion. Melanocytes, cells of melanoma origin, are specialized in releasing pigment vesicles, termed melanosomes. In melanoma in situ, we found melanosome markers in distal fibroblasts before melanoma invasion. The melanosomes carry microRNAs into primary fibroblasts triggering changes, including increased proliferation, migration and pro-inflammatory gene expression, all known features of cancer-associated fibroblasts (CAFs). Specifically, melanosomal microRNA-211 directly targets IGF2R and leads to MAPK signalling activation, which reciprocally encourages melanoma growth. Melanosome release inhibitor prevented CAF formation. Since the first interaction of melanoma cells with blood vessels occurs in the dermis, our data suggest an opportunity to block melanoma invasion by preventing the formation of the dermal tumour niche.
Publications:
Dror, Sanders et al. (2016) Nature Cell Biol., in press. (doi: 10.1038/ncb3399).
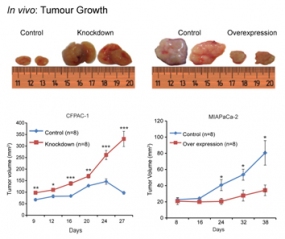
Two cell lines, which express miR-192 at high level (CFPAC-1) or low level (MIAPaCa-2) were transfected with constructs that suppressed or increased miR-192 expression, respectively. Xenografted into mice, strong effects on tumour growth were observed.
© dkfz.de
Pancreatic ductal adenocarcinoma (PDAC) is characterized by very early metastasis, suggesting the hypothesis that metastasis-associated changes may occur prior to actual tumor formation. We identified miR-192 as an epigenetically regulated suppressor gene with predictive value in this disease. miR-192 was downregulated by promoter methylation in both PDAC and chronic pancreatitis (CP), the latter of which is a major risk factor for development of PDAC. Functional studies in vitro and in vivo in mouse models of PDAC showed that overexpression of miR-192 was sufficient to reduce cell proliferation and invasion. Mechanistic analyses correlated changes in miR-192 promoter methylation and expression with epithelial-mesenchymal transition (EMT). Cell proliferation and invasion were linked to altered expression of the miR-192 target gene SERPINE1 that is encoding the protein plasminogen activator inhibitor-1 (PAI-1), an established regulator of these properties in PDAC cells. Notably, our data suggested that invasive capacity was altered even before neoplastic transformation occurred, as triggered by miR-192 downregulation. Overall, our results highlighted a role for miR-192 in explaining the early metastatic behavior of PDAC and suggested its relevance as a target to develop for early diagnostics and therapy.
Publications:
Botla et al. (2016) Cancer Res. 76, 4149-4159. [PDF]
MicroRNA variations in cells and their exosomes upon chemotherapy
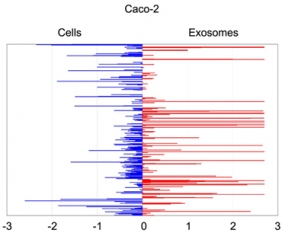
Quantitative asymmetric distribution of miRNAs in colorectal cancer cells and exosomes. Relative quantities (RQ) of the miRNAs amounts as isolated from exosomes were compared to those from the source cells Caco-2. Values are shown as log10 of RQ.
© dkfz.de
It has been convincingly proposed that exchange of molecules via exosomes is a means of eukaryotic intercellular communication, especially within the tumour microenvironment. However, there are no data on the alterations of exosomal molecular cargo upon pharmacological anticancer treatments. To approach this issue, we analysed the abundance of miRNAs and cancer-related proteins in exosomes secreted by Caco-2 (Cetuximab-responsive) and HCT-116 (Cetuximab-resistant) colorectal cancer cells before and after Cetuximab treatment. We also characterized both profiles in whole source cells.
Cetuximab significantly altered the molecular cargo of exosomes from Caco-2: we detected increased abundance of miRNAs and proteins that activate cell proliferation and proinflammatory processes; simultaneously, we observed a decrease of miRNAs and proteins related to immune suppression. These changes did not overlap entirely with those in source cells, suggesting a Cetuximab-linked distribution bias.
Molecular changes of a minor extent were also detected in exosomes from HCT-116. Transfection of exosomes from Cetuximab-treated Caco-2 into HCT-116 significantly increased HCT-116 viability; conversely, Caco-2 transfected with exosomes from treated HCT-116 did not show viability alterations. This suggests that the molecular phenotype of source cells is important for determining both the exosomal cargo as the biological effects of transferred exosomes.
Gene Ontology analysis of networks that comprise targets of differentially expressed exosomal miRNAs and proteins demonstrated a significant involvement of biological processes related to proliferation control, inflammation, immune response, and apoptosis. Our data shed light on molecular mechanisms of intercellular communication in eukaryotes.
Publications:
Ragusa et al. (2014) Oncoscience 1, 132-157. [PDF]