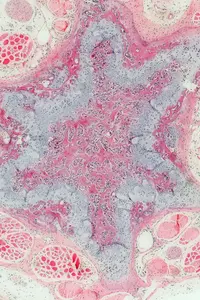
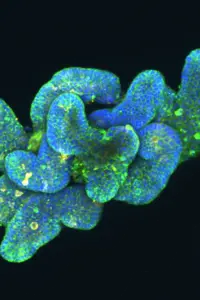
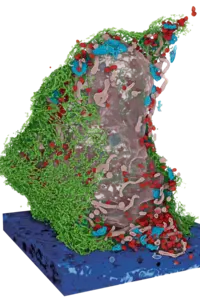
Research for a life without cancer
At the DKFZ, we want to ensure that fewer people develop cancer, that cancer can be cured or treated so effectively that those affected can live with the disease and grow old with a good quality of life.
About DKFZ







Latest from DKFZ
In recognition of Colorectal Cancer Awareness Month in March, the German Cancer Research Center (DKFZ) is drawing attention to the findings of a new study: Both regular stool-based tests and screening colonoscopies can significantly reduce the incidence of colorectal cancer and related mortality. When individuals participate consistently, the two prevention strategies are similarly effective.







Our research opens doors in the fight against cancer


Do you have questions on the topic of cancer?
Let us advise you!
Doctors from the Cancer Information Service answer your questions every day. Find out more now for free!
0800 - 420 30 40 daily from 8 a.m. to 8 p.m.