Brain Mosaicism and Tumorigenesis
- Functional and Structural Genomics
- Junior Research Group

Dr. Pei-Chi Wei
Principal Investigator
Not all brain cells have the same DNA. Some of these changes happen early in life and could play a role in the development of diseases like cancer. Our lab studies how these changes in DNA arise, how they are passed on to other cells, and how they spread in the brain.
Our Mission: Unraveling the Role of DNA Diversity in Brain Health
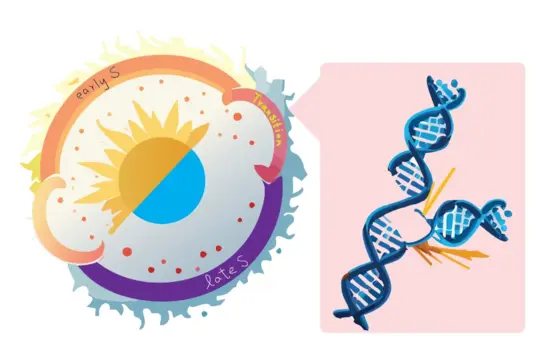
Brain Development is a Dynamic Process
The human brain is built from billions of neurons and astrocytes, generated through hundreds of divisions by neural progenitor cells. Remarkably, most of these cells form within just 24 weeks during fetal development. This process is carefully orchestrated in both time and space, shaping the ~400g newborn brain.
My lab explores fundamental questions: How do neural progenitor cells know when to stop dividing? Why and how do they extend their cell cycles? Does rapid cell division increase DNA damage in these cells? Understanding these mechanisms is key to uncovering how brain cancer begins, progresses, and evolves.
Methods and technologies
We have developed experimental and analytical tools to uncover the interactions between the cellular machinery responsible for copying and reading DNA sequences. Using high-throughput sequencing technologies, we measure the timing and speed of DNA replication and examine the transcriptional "traffic" created by the machinery decoding DNA.
Additionally, we create mouse models to investigate how neural stem cells compete for space and resources during early brain development. Our work also includes designing experimental systems that allow us to "travel back in time," tracing early developmental neural stem cell behaviors at later stages of life.
Goals and societal relevance
Brain cancer prevention faces significant challenges due to our limited understanding of how early cellular processes contribute to tumor formation. Our mission is to bridge this gap by uncovering how neural stem and progenitor cells drive brain development while maintaining DNA integrity. By studying the mechanisms of clonal expansion, cellular competition, and genomic stability, we aim to identify key risk factors and develop strategies for preventing brain cancers. Through this work, we strive to advance brain health and reduce the societal impact of neurological diseases.
Creativity and curiosity-drive interdisciplinary research

Our laboratory values individual perspectives in tackling scientific challenges, fostering a culture of collective creativity. We emphasize collaboration and open dialogue in a safe, judgment-free environment. We believe creativity thrives by combining personal experiences and knowledge, and it is enriched by drawing on the insights and expertise of others.
My lab explores various aspects of brain mosaicism, driven by curiosity and creativity in an interdisciplinary approach. Here are two examples of our research directions:
Do cells risk damaging their DNA as they divide?
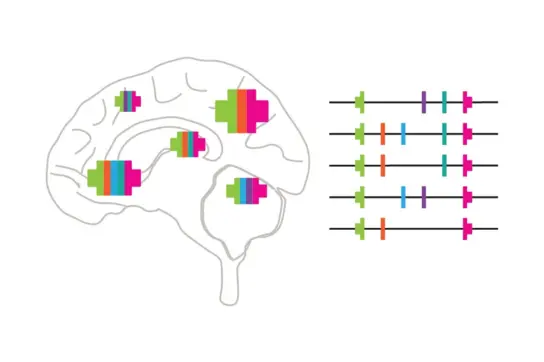
Our research focuses on understanding how DNA damage arises in neural stem cells and how this may contribute to brain disorders and cancers. Long genes, which control key neuronal functions like cell communication and plasticity, are especially prone to DNA damage. But why are these genes hotspots for such alterations?
We discovered specific regions in the DNA that frequently break, particularly in long genes essential for brain functions like communication and adaptability. These fragile regions become more vulnerable under stress during DNA duplication and are linked to neuropsychiatric disorders, cancers, and shared DNA changes in neurons originating from the same progenitors.
We found that these DNA breaks occur where copying DNA and reading its code collide. These areas lack the tools to repair stalled copying processes, making them especially prone to damage. Using advanced techniques, we showed that these breaks often align in ways that promote large deletions in critical genes.
We are investigating whether this DNA damage plays a role in diversifying the genomes of neural founder cells and whether deletions in critical genes contribute to tumor development.
How do neural stem cell clone sizes influence brain complexity?
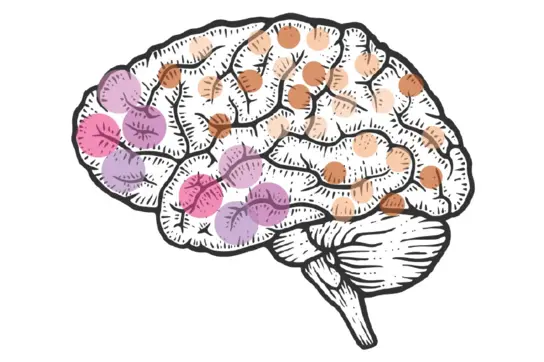
Our research delves into how the size of neural stem cell clones impacts the brain’s structure and function. Neural stem clones are groups of neurons that share the same DNA blueprint, and their size directly influences the spatial organization and functional diversity of neurons in the brain. Intriguingly, studies show that the size of neuronal clones varies across brain regions, suggesting that clone size plays a key role in tailoring the brain’s architecture to its specific needs.
My lab develops preclinical tools and models to investigate clonal expansion, selection, and evolution in the developing brain. We focus on identifying the triggers of clonal competition—a process that can drive unregulated, excessive cell proliferation, also known as precancerous hyperproliferation.
Our goal is to determine whether such unchecked proliferation in normal tissue increases the risk of cancer development. Ultimately, this research could uncover new risk factors and inform strategies for preventing brain cancer.
Current team
-

Dr. Pei-Chi Wei
Principal Investigator
-
Jana Berlanda
-

Lorenzo Corazzi
Ph.D. student
-

Giulia Di Muzio
Ph.D. student
-

Boyu Ding
Ph.D. student
-

Marco Giaisi
Lab Manager
-
Hsin-Jui Lu
-
Anna Marx
-

Li-Chin Wang
Data scientist
Associated team members
Michael Allers
M.D. student with Prof. Dr. Dr. Peter Huber (E055)
E-mail: m.allers(at)dkfz-heidelberg.de
Phone: +49 6221 42 3249
Selected Publications
Lorenzo Corazzi, Vivien S Ionasz, Sergej Andrejev, Li-Chin Wang, Athanasios Vouzas, Marco Giaisi, Giulia Di Muzio, Boyu Ding, Anna J M Marx, Jonas Henkenjohann, Michael M Allers, David M Gilbert, Pei-Chi Wei
Aseda Tena, Yuxiang Zhang, Nia Kyritsis, Anne Devorak, Jeffrey Zurita, Pei-Chi Wei (co-correspondence), and Frederick W. Alt
Wei PC, Chang AN, Kao J, Du Z, Meyers RM, Alt FW, Schwer B
Get in touch with us

