Information Technology
Dr. Claudia Galuschka


At DKFZ, Information technology (IT) is a basic need for the work of all employees. This is due, in particular, to modern laboratory techniques in the field of genome analysis as well as radiological image processing with their large data volumes on a petabyte scale. In order to gain significant information and knowledge from research data, elaborate technologies are necessary. But also the penetration of everyday work with communication technology and office applications is still increasing, as facility monitoring and more and more administrative processes are being digitized. The many aspects of IT security play an important role at DKFZ because processing of personal data takes place in many areas and is required in many national and international scientific projects and co-operations. Therefore not only confidentiality and integrity of the data and applications are important, but also the availability is essential for good co-operation. IT thus has a leading role as an enabling technology for the life sciences and especially for cancer research.
The Information Technology Core Facility (ITCF) is responsible for optimum usage of IT at DKFZ, for supporting staff of all departments in matters of choosing IT tools for their special needs as well as for providing central IT Services and support which are essential for everyone at DKFZ. Individual solutions are also supported in planning and operation. This is done on a service-oriented basis. All essential strategic and operational functions and tasks at DKFZ are substantially facilitated by the ITCF.
Working groups

The Application Development working group develops applications for all areas of the DKFZ. These are primarily web applications in connection with databases hosted on the central servers of the IT Core Facility. We also create the appropriate APIs (programming interfaces) to enable data exchange with third-party programs at the source code level.
The working group Desktop Services runs the service centres for user enquiries and works on matters relating to the desktop environment as well as mobile devices and operates the high quality printing centre.

The IT Security working group is responsible for the operational monitoring of IT security at the DKFZ as well as for the introduction, further development and operation of services and processes for the prevention of and response to IT security incidents (Computer Emergency Response Team -CERT).

The network group is responsible for the entire data network infrastructure of the DKFZ and its sites. In addition to the classical LAN connection of a workstation, this also includes the coverage and provision of a comprehensive wireless LAN environment.
The group also designs and maintains the network infrastructure of the different data centers and their links and interconnections. Which also includes security services and firewall zone transitions, as well as Internet connectivity and partner site integration.
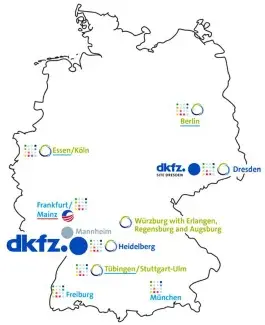
The Partner Sites working group supports the translational sites and networks of the DKFZ (DKFZ-Dresden, DKTK, HECTOR, HI-TRON, NCT extension) in IT-relevant issues as well as in planning, setting up and operating the IT connection to the core center in Heidelberg. In addition, the working group is the contact for the coordinators at the sites and the central coordination offices of the partner sites at the DKFZ.

The working group Server and Compute operates central platforms und infrastructures for scientific computing (SCI). This currently includes the operation of the DKFZ Cloud based on Openstack, the cloud infrastructure for the german network for bioinformatics infrastructure de.NBI and in close collaboration with the ODCF a cluster for general-purpose computing on graphics processing units (GPGPU). Central servers, mail and file service, printing services, data storage and scientific computing are the tasks of the working group.

Central software systems and services are provided in the working group Software Systems. This includes user management, software distribution and license monitoring, authorization and resource management in file service, database service, document management and the electronic labbook.
Management-Team
-
Dr. Claudia Galuschka
Head of Department
-
Jürgen Trauth
WG Application Development
-
Michael Cop
WG Desktop Services
-
Jan Tölken
WG IT Security
-
DKFZ CERT
WG IT Security
-
Nils Kückenhoff
WG Network
-
Heidrun Binder
WG Partner Sites
-
Markus Hohenhaus
WG Server and Compute
-
Heiko Rosenfelder
WG Software Systems
TÜV IT certification
For DKFZ, security of IT and data is of highest priority. Therefore a certification of the main data center has been done by TÜV Informationstechnik GmbH. This includes environmental conditions, fire detection and fire-fighting technology, safety and access systems, energy supply, air-conditioning and organization.
The data center has been certified to Trusted Site Infrastructure TSI 3.2 Level 1 (enhanced). For the elements involved in security and organization, all the criteria of the catalog were fulfilled, which also correspond to a higher level.
A corresponding certification will also be required for all other data centers which are put into operation.
Further information on this topic:
Internet access with "eduroam"
Eduroam is a worldwide bond of university and research networks. It enables its members in many parts of the world, especially in Europe, to easily access the Internet via WLAN using their personal user ID. The WLAN identifier (SSID) is uniformly called "eduroam".
Every DKFZ employee with a valid user ID is entitled to use eduroam. To connect to the ssid „eduroam", it is essential to install the profile.
Windows, Android, iOS: https://www.geteduroam.app/ macOS: https://cat.eduroam.org/
If the profile is installed, make sure that all old eduroam configurations have been deleted beforehand. You can find detailed instructions for each operating system under the „Installation Instructions" tab.
The „eduroam" service only gives you access to the Internet. Access to the DKFZ intranet is only possible if you establish a VPN connection. The configuration of the VPN access on your computer can be found on the intranet in the ITCF Wiki.
An overview, as well as lists of the affiliated locations, are available under the following link for Germany, Europe, Canada and the USA. However, these lists do not include all locations where eduroam is available:
Users of iPhone, iPad and Android devices can also use the "eduroam Companion" app to find eduroam locations. This app can be found in the respective app stores. However, the DKFZ does not support "eduroam Companion" or Android devices in general.
Get in touch with us

ITService
Core Facility ITPostal address: