Mammalian Cell Cycle Control Mechanisms
- Immunology, Infection and Cancer

Prof. Dr. Ingrid Hoffmann
Group Leader
The research group Cell Cycle Control and Carcinogenesis is studying the molecular mechanisms underlying centrosome duplication.

Our Research
Initiation and propagation of cancer is not possible without cell division (mitosis) which depends on small cellular organelles known as centrosomes. Similar to DNA replication, the centrosome is duplicated only once within a normal cell cycle. Having the correct number of centrosomes is crucial for proper chromosome segregation during cell division and for the prevention of genomic instability, a hallmark of many cancer cells. Failure to properly duplicate centrosomes results in supernumerary centrosomes, which are frequently found in tumors. The research group Cell Cycle Control and Carcinogenesis is studying the molecular mechanisms underlying centrosome duplication. The aim is to identify and characterize proteins that regulate this process. Our focus is placed on the key regulator of centriole duplication, the polo-like kinase Plk4.
Other projects in the lab are aimed at
- understanding how misorientation of the mitotic spindle contributes to cancer development
- unraveling the mechanisms of drug resistance that imposes a major problem in cancer therapy.
Projects
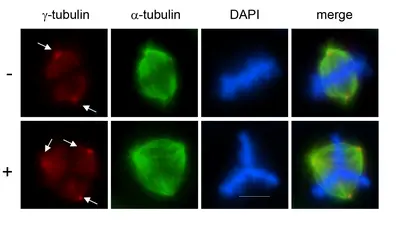
Failure to properly control centrosome number results in supernumerary centrosomes, which are frequently found in cancer cells. Centrosomes are small organelles that contain a pair of barrel-shaped centrioles surrounded by pericentriolar material, PCM. Centrioles duplicate once during the cell cycle to give rise to two mitotic spindle poles, each containing one old and one new centriole. Centrosome duplication must occur in coordination with other cell cycle events, including DNA synthesis.
Most human cancers exhibit centrosome duplication errors which might lead to aneuploidy and cancer formation. How centrioles are assembled and how their numbers are controlled within cells constitute long-standing unresolved questions.
Plk4, a polo-like kinase family member, is the key regulator of centriole duplication. We are studying the pathways underlying Plk4-induced centriole duplication in normal and malignant cells by identifying and characterizing substrates and regulators of Plk4.
To decipher signaling pathways involved in centrosome overduplication in cancer cells we use HPV (human papilloma virus)-induced cervical carcinogenesis as a model system. The high-risk human papillomavirus type 16 E6 and E7 oncoproteins cooperate to induce mitotic defects and genomic instability by uncoupling centrosome duplication from the cell division cycle. Expression of the E7 oncoprotein rapidly drives centrosome duplication errors leading to aberrant centrosome numbers. The goal of our study is to decipher the cellular pathways and mechanisms of action of the high-risk HPV16-E7 oncoprotein leading to centrosomal abnormalities and subsequent genomic instability.
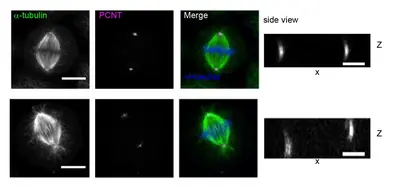
Proper positioning of the mitotic spindle axis within the cell is a fundamental process in development and stem cell division. In symmetrically dividing cells, precise spindle orientation and positioning ensures equal distribution of cellular components. In contrast, in asymmetrically dividing cells, accurate orientation and placement of the mitotic spindle away from the center of the cell results in cell fate diversity. In most epithelia, cells divide symmetrically and orient their mitotic spindle parallel to the apical-basal surface, ensuring expansion of the epithelial sheet with side-by-side growing daughter cells. Any misregulation in spindle orientation can result in disorganized tissue morphology due to cell multi-layering, which could be associated with earliest cancer developments.
Our aim is to analyze cellular pathways underlying spindle orientation and how spindle orientation crosstalks with the extracellular matrix (ECM) to induce tumor invasion and metastasis.
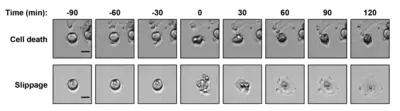
Spindle poisons are drugs that prevent the assembly (nocodazole, vincristine) or the disassembly (taxol or paclitaxel) of microtubules. They impair spindle function and chromosome segregation during mitosis by preventing normal microtubule dynamics. Treatment with spindle poisons therefore activates the spindle assembly checkpoint (SAC) leading to mitotic arrest. Those cells frequently undergo mitotic cell death. The microtubule poisons paclitaxel or vincristine are commonly used in chemotherapy in order to prevent proliferation of cancer cells by inducing cell death. Cells that are arrested in mitosis sometimes evade mitotic cell death. Instead these cells leave mitosis without completing a normal cell division and become tetraploid. This phenomenon is called mitotic slippage. During chemotherapy with spindle poisons cancer cells may evade mitotic cell death by performing mitotic slippage. This in turn leads to chemoresistance of cancer cells which continues to be a major impediment in medical oncology.
The goal of this project is to decipher the mechanism underlying chemoresistance (mitotic drug resistance) by analyzing the regulation of the F-box protein and tumor suppressor Fbxw7 during mitosis.
Selected Publications
Zhu, M., Settele, F., Kotak, S., Sanchez-Pulido, L., Ehret, L., Ponting, C, Gönczy, P. and Hoffmann, I.
Kschonksak, Y. and Hoffmann, I.
Richter, K., Kschonsak, Y., Vodicska, B., and Hoffmann, I.
Grossmann J, Kratz AS, Kordonsky A, Prag G, Hoffmann I. (2024)
Selection of Recent Publications
Open Positions
The German Cancer Research Center has an international PhD Program with 36 fellowships granted annually. The program provides interdisciplinary training and research opportunities in the field of Biology and Cancer Research. Intested candidate might contact the group leader for further information.
For details about the application visit Helmholtz International Graduate School for Cancer Research at DKFZ.
Get in touch with us
