Success Stories
Vaccine against cervical cancer
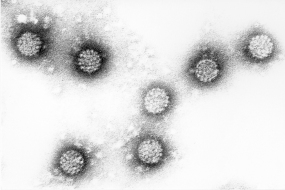
HPV_1
© DKFZ/Prof. Dr. H. Zentgraf
The innovative vaccine against cervical cancer marks a milestone in the prevention of what is the second commonest cancer in the world and the third-ranking cause of death in women. More than 4,500 women a year in Germany still contract cervical cancer and around 1,500 die from the disease. If all girls were to be vaccinated in time, these figures could be drastically reduced. In 2014, the Standing Vaccination Committee at the Robert Koch Institute recommended lowering the age for HPV vaccination to between 9 and 14, so that more girls than ever can be protected from the infection before they become sexually active. Moreover, at this younger age only two vaccinations are required to afford lifelong protection. The vaccine costs are reimbursed by the health insurers.
Two vaccine products have now received market approval in over 50 countries: Gardasil® from Merck & Co and Cervarix® from GlaxoSmithKline (GSK). Together they produce annual sales in the billions. Both vaccines provide almost complete cover against HPV types 16 and 18, which are responsible for around 70 percent of all cases of cervical cancer. Since Gardasil is also targeted against HPV 6 and HPV 11, which cause other genital disorders such as genital warts (condylomata), approximately 90 percent of these cases can be prevented by vaccination. A successor product, Gardasil 9, has now been approved by the US and European regulatory authorities and has now been available on the German market since 2016. In addition to HPV types 6, 11, 16 and 18, Gardasil 9 is also targeted against five other types (31, 33, 45, 52 and 58), which account for around 20 percent of all cases of cervical cancer.
Links:
https://www.krebsinformationsdienst.de/vorbeugung/risiken/hpv-impfung.php
High-precision radiosurgery of tumors
CyberKnife® / iris collimator
© DKFZ/Marco Müller
"Accuray was a logical choice for us in selecting a research partner given our longstanding collaboration on the CyberKnife® System and the company's leadership in imaging systems and motion management", said Professor Wolfgang Schlegel on the conclusion of the collaboration agreement with the American company. The joint projects also include, in close cooperation with Heidelberg University Hospital, researching and expanding intensity-modulated radiation therapy (IMRT) and the treatment options for Tomotherapy® - an Accuray technology for reducing the exposure to side effects during the radiotherapy of tumor patients.
Links:
Breaking the diffraction barrier
© DKFZ/Abt. Optische Nanoskopie
In the "Optical Nanoscopy" Laboratory at the DKFZ – and in combination with an ultrafast electro-optical scanner developed by the engineer Dr. Jale Schneider at RWTH Aachen University - an STED nanoscope has been developed, under the direction of Stefan Hell and Johann Engelhardt, that enables images to be recorded in intervals of milliseconds. This is several thousand times faster than has previously been possible with laser scanning microscopes. As a result, dynamic processes within the cell that had not been accessible to optical analysis to date can now be examined in detail.
Links:
http://www.nobelprize.org/nobel_prizes/chemistry/laureates/2014/hell-lecture.html
Innate Cell Engagers improve the anti-tumor activity of immune cells

TandAb® model
© Affimed 2014
ICE® activate innate immune cells, such as NK cells or macrophages, by binding to their receptor CD16A and simultaneously to specific antigens on the tumor cell surface. Once these connections are established, the innate immune cells become engaged and induce tumor cell killing via antibody-dependent cell-mediated cytotoxicity (ADCC) and antibody-dependent cellular phagocytosis (ADCP).
AFM13, the company's first-in-class ICE®, has demonstrated clinical activity in Phase 1 and Phase 2 clinical trials, e.g. when used as monotherapy in CD30-positive lymphomas and Hodgkin lymphomas. In a study with The University of Texas MD Anderson Cancer Center, AFM13 demonstrated an unprecedented efficacy, when used in combination with allogenic NK cells in patients with CD30-positive Hodgkin lymphoma. In combination with the anti-PD-L1 antibody pembrolizumab in patients with Hodgkin lymphoma, Phase 1 clinical trials resulted in higher response rates than the individual treatments alone.
AFM24 targets EGFR on cancer cells. This allows targeting of EGFR-expressing solid tumors. AFM24's mode of action of does not rely on EGFR signal inhibition, and only uses the tumor antigen as a docking site, which differentiates itself from previous EGFR-targeting therapeutics and potentially offers a novel option for patients who have developed resistances to EGFR signal inhibitory treatments. AFM24 is investigated as monotherapy and in combination with a PD-1 inhibitor or adoptive NK cells.
Furthermore, AFM28, a CD123-targeting ICE® for the treatment of patients with AML, has entered its first clinical study in 2023.
In 2018 Affimed introduced the ROCK® (Redirected Optimised Cell Killing) platform, a tool that allows a modular development of novel ICE®. Several molecules are in preclinical development with promising initial results.
Affimed collaborates with global pharmaceutical and biotechnology companies to improve cancer treatments across a broad range of tumor types. Besides a partnership with Genentech, Roivant Sciences, Merck Inc., Roche as well as a number of academic institutions collaborate with Affimed to bring new options to patients in need.
Links:
https://www.affimed.com
Active substance against the most malignant brain tumor

A glioblastoma in the MRT
© DKFZ/Med. Physik in der Radiologie, Foto: Michael Bock
In preclinical tests, Apogenix was able to show that APG101 restores the formation of blood cells (erythropoiesis) in patients suffering from myelodysplastic syndromes (MDS). MDS is an anemic bone marrow disorder associated with the risk of developing into an acute myeloid leukemia, a cancer of the blood. Apogenix then conducted a phase I clinical trial with APG101 in the treatment of transfusion-dependent MDS patients with severely impaired erythropoiesis in order to test the safety, efficacy and tolerability of the drug. The results of the study are expected in the summer of 2016.
APG101 possesses Orphan Drug Status for the treatment of gliomas in the EU and for the treatment of glioblastomas and MDS in the USA. The company CANbridge Life Sciences (Beijing, China) is responsible for the licensing and marketing of APG101 in China, Macao, Hong Kong and Taiwan. Thanks to this partnership, Apogenix can earn revenues that can then be used, in turn, for its own development in the other countries.
Links:
http://apogenix.com/files/pdfs/DE/20160615_Apogenix_MDS_Topline_Data_DE.pdf
Software for improved diagnosis and therapy planning
© Mint Medical GmbH
The company has introduced a certified quality management system and applies this to its products, which are both compliant with medical devices legislation and CE-tested. "Mint Liver" was developed as a tool specifically for the computer-aided diagnosis and therapy planning of liver diseases. It can provide a 3D reconstruction of a patient's liver, simulate various surgical procedures and help determine the optimal strategy for the surgical procedure even before the operation and, if necessary, enable the strategy to be quickly adapted to new findings during the operation.
The software development mint LesionTM facilitates the efficient monitoring and follow-up of cancer treatments. As well as providing quantitative and qualitative statements on the development of tumors during the course of treatment, Lesion™ can also be used to produce assessments according to the established standards in radiology simply, quickly and flexibly. Mint Medical products are now used worldwide in routine clinical practice for cancer screening, cancer staging and for assessing the response to cancer treatments. During clinical trials, LesionTM supports the reporting process in accordance with the criteria. It can be individually configured for case-specific, multicenter clinical trials. As a Clinical Trial Management System that has been approved by the FDA, mint LesionTM can support project management, study-compliant read procedures and data management in the implementation of clinical trials for imaging CROs and pharmaceutical companies. For imaging biomarker analysis, mint LesionTM can cover the whole workflow, including all the associated tasks of imaging biomarker research.
The CEO of Mint Medical GmbH, Dr. Matthias Baumhauer describes the company's vision as follows: "Mint Medical products assign a central role to the patient as an individual in our healthcare system. We therefore believe that our solutions will be an important component of any advanced hospital in the medium term."
Links:
Oncolytic viruses to treat brain tumors
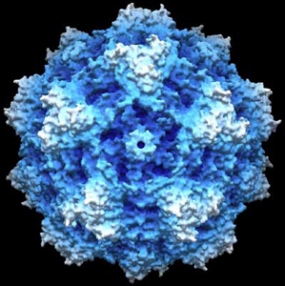
Computer simulation of a parovirus
© DKFZ / Antonio Marchini
Since the preclinical studies needed to progress the project through to clinical use are very time-consuming, a partner was sought and eventually found in the company Oryx GmbH & Co KG. Oryx is specialized in taking research projects in cancer medicine through the preclinical and early clinical development stages and selling them to the pharmaceutical industry. In January 2008, the Munich-based company signed a cooperation agreement with the DKFZ and Heidelberg University Hospital, which is also involved in the development of viral therapy. Working together with industrial partners, Oryx coordinated the large-scale technical production and subsequent pharmacology and toxicology testing of the therapeutic viruses as well as the approval procedure with the Paul Ehrlich Institute. Clinical phase I/IIa trials began in the Fall of 2011 –the first time that brain tumors were treated with viruses in Europe. On 12 June 2015, Oryx announced the successful completion of the clinical phase I/IIa trial on the treatment of patients with progressive primary and recurrent glioblastoma with H1 parvoviruses, in which the vaccine was found to be safe. The oncolytic parvoviruses will now be tested on other malignant tumors, including pancreatic cancers.
Links:
https://www.dkfz.de/de/presse/pressemitteilungen/2011/dkfz-pm-11-56-Mit-Viren-gegen-Hirntumoren.php
Early detection of cervical cancer
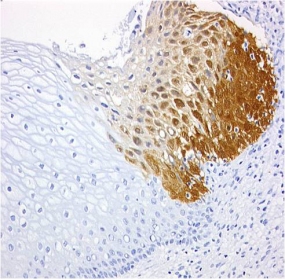
CINtec® p16 histology of a zervix carcinoma
© mtm-laboratories
The basis for the patent-protected test kits was the company's own antibody clone E6H4TM, which had been developed specifically for immunochemical applications. The antibody is used to detect the overexpression in cervical cells of the cyclin-dependent kinase inhibitor p16INK4a, an established biomarker for the oncogenic activity of high-risk human papillomaviruses (HR-HPV), which can then trigger cervical cancer. The detection of this biomarker served as the basis for in-vitro diagnostic products developed by mtm laboratories, including the CINtec® Histology Kit for use on slides with formalin-fixed, paraffin-embedded sections from cervical biopsies, and the CINtec® Cytology Kit for use on smears and cell samples in a liquid medium. mtm laboratories optimized this cytology test and marketed the resulting product, the CINtec®PLUS Cytology Test, which represents a dual staining of the biomarker p16 and the proliferation marker Ki-67. The co-expression of these proteins indicates a transforming HPV infection is present and the woman is at elevated risk for having high-grade cervical neoplasia
This test offers a high level of sensitivity and specificity in the detection of high-grade cervical disease. The clinical benefit of these test kits has been demonstrated and validated in many large-scale studies. They proved to be far more precise in diagnosing cervical cancer and its precursor stages than traditional tests, including the classical cancer test with smear specimens that has been used in millions of patients, the Pap test developed by Georgios N. Papanicolaou.
In 2011, Roche announced the acquisition of mtm laboratories, which became part of Roche's Tissue Diagnostics (Ventana Medical Systems Inc.). Roche bought mtm for an upfront payment of 130 million EUR plus 60 million EUR upon reaching performance-related milestones. Most recently, in 2020, Roche received the FDA approval of the CINtec®PLUS Cytology Test to aid clinicians' efforts in preventing cervical cancer. FDA considered data from the Roche-sponsored IMPACT (IMproving Primary screening And Colposcopy Triage) trial, which enrolled approximately 35,000 women in the U.S. to clinically validate CINtec PLUS Cytology as a triage test in various screening scenarios. Prior to FDA approval, the CINtec PLUS Cytology test had been used as a triage test for HPV-positive results and mildly abnormal Pap cytology results in Europe, Asia, South America, Canada and Australia. Roche's Cervical Cancer Portfolio covers the entire spectrum of screening, triage, and diagnostic solutions for cervical cancer and constitutes a key factor in the success of WHO's worldwide program to eradicate cervical cancer by 2030.
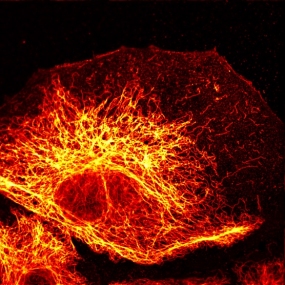
Cell type diagnosis of metastases using antibodies to cytoskeletal proteins
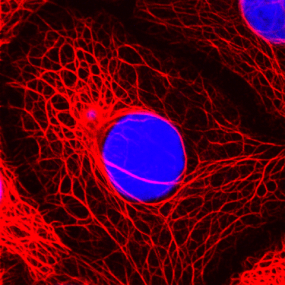
The cytokeratin skeleton of breast cancer cells
© DKFZ / Lutz Langbein
In order to meet the worldwide demand for antibodies to cell type-specific forms of intermediate filaments for use in diagnosis and biomedical research, Franke, together with the DKFZ immunologist Professor Günter Hämmerling and two other colleagues from Heidelberg University, formed the company PROGEN Biotechnik GmbH in 1983, one of the very first biotechnological companies in Germany. Immunodiagnosis with antibodies against cytoskeletal and desmosomal proteins has now become an integral part of every molecular pathology laboratory. In October 2012 PROGEN Biotechnik GmbH became a 100% subsidiary of the R-Biopharm AG, under whose umbrella PROGEN continues to supply the existing market and break into new markets.
In 2019 PROGEN acquired the exclusive rights from DKFZ for our AAV (Adeno-Associated Virus) antibodies and products based on these AAV antibodies. AAVs are small viruses of the parvoviridae family. AAV derived vectors have gained interest in the field of viral based gene therapy due to several advantages. Amongst others, AAV is known to be non-pathogenic. The absence of any symptoms or human disease being associated with AAVs makes AAV derived vectors a perfect vehicle for the delivery of genetic material to human cells. In the last decade, recombinant AAV (rAAV) vectors have been intensively studied and used for in vivo gene transfer, representing a promising therapeutic approach to target inherited, genetic diseases and many others. In this context, a transgene is inserted into the viral genome, thereby replacing the viral genes for replication and packing. After purification, the recombinant virus can be applied to the patient, transducing the target cells and restoring the defective or missing gene of interest. AAV has been demonstrated to enable long-term transgene expression due to its latent state within the cell. The derived vectors rarely integrate into the genome but reside as episomes in the nucleus, lowering the chance of spontaneous gene alteration. Furthermore, AAV naturally infects a wide range of cell and tissue types. However, the tissue tropism of the different AAV serotypes is determined by the different cell surface receptors used for the attachment to the target cell. As of April 2023, rAAV vectors have been used in more than 300 clinical trials worldwide (https://clinicaltrials.gov). The range of diseases treated with rAAV based gene therapeutics includes inherited diseases like cystic fibrosis, haemophilia B and muscular dystrophy, as well as acquired diseases like severe heart failure and Parkinson´s disease. Some of these AAV derived gene therapies have shown promising results so far and three AAV gene therapies against inherited retinal dystrophy, spinal muscular atrophy and Hemophilia B have already been approved by the FDA.
Starting as a pioneer antibody manufacturer, PROGEN has become a globally operating biotech company and a reliable partner for academia, biotech and pharma. The company developed from an antibody manufacturer to a leading supplier and exclusive provider of AAV antibodies and analytical tools for accurate and reliable AAV titer determination supporting the development of safe and efficient gene therapies. The team consists of life science and AAV experts, many of them DKFZ alumni, and is partnered with specialists within the field of gene therapy and the life sciences worldwide. The company follows their mission to provide high quality products that help advancing new therapies and develop existing research processes safely, quickly and affordably for the life science community.
Links:
http://www.dkfz.de/de/helmholtz-zellbiologie/Franke-in-Cancer_Research_DKFZ_2014_web.pdf
A radionuclide-coupled substance for the diagnosis and treatment of prostate cancer
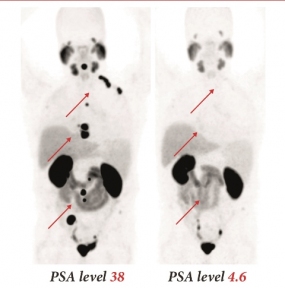
PSMA-617 mark of a prostate cancer patient
© DKFZ / Matthias Eder
Antibody microarrays for screening of biomarkers in cancer

© Sciomics GmbH
In 2013, Hoheisel and his colleague Dr. Christoph Schröder, formed the biotech company Sciomics GmbH in Heidelberg, which uses its protein microarrays to offer services for medical research, diagnosis and industry including, in particular, biomarker screening, the verification of biomarker candidates, the analysis and localization of drug targets and their signaling pathways and the characterization of antibodies. Schröder is the CEO of Sciomics GmbH, while Hoheisel still acts as a consultant for the company.
The research team at the DKFZ headed by Hoheisel and Schröder has used microarrays from cancer-relevant antibodies to identify, file for patenting and publish a series of biomarker signatures. These microarrays may be important for the diagnosis, prognosis and prediction of cancer. Predictive biomarkers provide information about the possible effect of the therapeutic intervention, whereas prognostic biomarkers deliver information about the patient's illness and its course independently of the treatment. Among other aspects, they are investigating the altered plasma protein composition in patients with chronic lymphatic leukemia and other B-cell lymphomas, as well as cancer-associated proteins in pancreatic carcinoma, a type of cancer which, to date, has proved difficult to treat and usually diagnosable too late. In another study, the scientists have used an antibody microarray to predict recurrences of bladder cancer, which reappears within five years of surgical removal of the tumor in around 60 percent of patients. In their comparison of patients with and without recurrence, they have identified 255 proteins that are present in the tumor in small or large quantities. Twenty of these proteins produced a pattern which, with 100 percent specificity and 80 percent sensitivity, represents a highly promising candidate as a predictive biomarker signature for relapses of bladder cancer.
Links:
Strategic partnership with Bayer Pharma to develop innovative cancer drugs
Tumor sample, imbedded in paraffin
© Bayer HealthCare AG
In 2013, the alliance was expanded to include the field of immuno-oncology, and the corresponding researchers at BHC and the DKFZ are working together on more advanced projects in a Joint Immunotherapeutics Lab in Heidelberg. Up to 2.5 million euros a year is being invested in these projects. In total, the two partners will be investing up to six million euros a year in the collaborative oncology research projects for the period 2014 to 2018. If any projects prove successful, the DKFZ will receive a share of the revenues. Of the more than 30 projects initiated to date, many have already reached important milestones and progressed to the next phase of drug development, namely compound screening to identify new potential drug candidates.
Links:
Competency cluster in imaging and radiotherapy: the alliance with Siemens Healthcare
7 Tesla magnetic resonance tomograph
© DKFZ / Tobias Schwerdt
For example, Dr. Heinz-Peter Schlemmer's team of the Radiology division at DKFZ is focusing on improving prostate cancer diagnostics by combining magnetic resonance imaging (MRI) with ultrasound, while the combination of MRI with positron emission tomography (PET) can provide quantifiable information on the metabolic activity of the tumor and its malignancy. Such methods can improve the accuracy of biopsy sampling by making sure that the biopsy needle precisely targets the site of interest. Moreover, they have increased the performance of 68Ga-PSMA PET/MRI by improving the software that analyzes the results of the scan. PSMA is a membrane surface antigen in prostate tissue that can be traced by PET imaging, but is prone to generating image artifacts. Dr. Kachelrieß's team in the X-Ray Imaging and Computed Tomography division has introduced an algorithm that can recognize and remove such artifacts, and thereby improve the accuracy of diagnosis based on PET/MRI scans.
More precise structural and functional imaging in cancer diagnosis is achieved with the 7 Tesla high field MRI scanner supplied by Siemens and housed in a dedicated building specially constructed by the DKFZ with steel shielding in order to protect surrounding electronics from the strong magnetic field. The high magnetic field strength not only produces stronger signals, but can also be used to create innovative image contrasts to provide information about, for example, oxygen consumption and individual metabolic products in the tumor tissue. This allows conclusions to be drawn about the malignancy of the cancer. Brain tumors, for example, can be diagnosed and characterized with hitherto unachievable precision. In 2019 Mark E. Ladd, Ph.D., Head of the Division of Medical Physics in Radiology at DKFZ, was a finalist for the German Future Prize alongside his cooperation partners, Christina Triantafyllou, Ph.D. from Siemens Healthineers, and Professor Arnd Dörfler, M.D., Head of University Hospital Erlangen's Department of Neuroradiology. They received the nomination for the development of the first ultra-high field 7 Tesla MRI scanner for clinical use. This technology is able to accurately image anatomical structures as small as 0.2 mm, allowing early diagnosis of not only tumor lesions but also neurological diseases like multiple sclerosis (MS), epilepsy and Parkinson's disease.
For treatment with protons and heavy ions, the scientists in the alliance are developing mathematical techniques for optimizing and accelerating radiotherapy. In October 2012, the Heidelberg Ion Beam Therapy Center, together with the Helmholtz Center for Heavy Ion Research in Darmstadt, commissioned a gantry, the world's only such facility, to form a gigantic radiation unit that can irradiate tumors with millimeter precision from any angle with heavy ions and protons. The scientists in the DKFZ – Siemens Healthineers alliance have since been involved in developing software, generating treatment plans and performing clinical trials for testing the effectiveness of the heavy ion and proton irradiation in various tumors.
The efficiency and safety of heavy ion irradiation therapies rely on the precise identification of the tumor site, but the current clinical standard method, single-energy CT scans, has weaknesses. To improve accuracy of high-energy beams, researchers of the DKFZ – Siemens cooperation together with colleagues from OncoRay (Dresden) have worked on implementing dual energy CT (DECT) into clinical usage. DECT can provide two images based on two x-ray energies simultaneously that enable a more precise, patient-specific targeting of the ion beam. After the initial success of the cooperation, the team developed a clinically certified software that made this application of DECT available for clinicians at other particle therapy centers.
Since 2019, the partners in the alliance also cooperate in a BMBF-funded project to investigate the use of MRI in particle therapy. Within this project, two MR scanners have been implemented at the HIT facility: one for offline imaging of patients just outside the gantry treatment room and one for experimental purposes in the experimental beam-line. The team is also investigating novel software solutions to directly use MRI for particle therapy treatment planning.
In 2022, the newest generation linear accelerator for radiation therapy from Varian (a subsidiary of Siemens Healthineers) and a 3 Tesla MRI system from Siemens were installed in adjacent rooms in a common bunker of the DKFZ. The two machines are connected by a dedicated patient shuttle, enabling unique research studies for MR-guided radiotherapy. The soft-tissue contrast of MRI provides better delineation of tumor tissue and will enable personalized therapy adapted to changing organ positions and metabolic characteristics of the tumor.
Links:
https://www.siemens-healthineers.com/press/releases/pr-20190911034shs.html
https://www.siemens-healthineers.com/de/news/ionenstrahltherapie.html
https://www.dkfz.de/en/presse/pressemitteilungen/2017/dkfz-pm-17-35c-Double-imaging-of-prostate-cancer.php
Detecting and combating cancers through cancer stem cell research
Tumor stem cells of pancreas cancer
© DKFZ / A. Trumpp
The HI-STEM gGmbH was co-founded by the DKFZ and the Dietmar Hopp Foundation with the goal to create a dedicated public-private research institute that focuses on tumor stem cell research and guides clinical translational of the latest discoveries in this field. This includes the development of diagnostic tools, and research for innovative therapies to target tumor stem cells in leukemias and solid tumors. The institute is located on the fourth floor of the DKFZ main building in Heidelberg and is directed by Professor Dr. Andreas Trumpp, who is also the head of the Division of Stem Cell and Cancer at the DKFZ. HI-STEM closely interacts with other Heidelberg and national based Institutions/networks, such as the DKFZ, the National Centers for Tumor Diseases (NCTs), Heidelberg University Hospital, the German Stem Cell Network, and the BioRN, a life science research and industry cluster in the Rhein-Neckar region. HI-STEM currently consists of seven research groups including junior research groups. Their main research focus can be specified into three main areas: (1) Hematopoietic and Leukemic Stem Cells, (2) solid and metastasis Cancer Stem Cells (Breast-, Colon- and Pancreatic cancer) and (3) regenerative therapies for degenerative diseases using stem cell reprogramming.
Hematopoietic stem cells (HSCs) located in the bone marrow are typically dormant, have long-term self-renewal activity and control live long blood production by generating proliferative progenitors that differentiate into functional mature blood cells. HI-STEM characterized the molecular mechanisms mediating long-term self-renewal and stemness down to the single cell level and showed that the state of dormancy is protecting the genomic integrity of HSCs. This is critical as mutations in single HSCs lead to clonal hematopoiesis caused by mutant HSCs with a clonal growth advantage. Clonal hematopoiesis becomes quite common with higher age and is associated with increased risk of cardio-vascular diseases such as cardiac infarction due to mutated macrophages generated by mutated HSCs. Additional mutations in this clone may then lead to the generation of leukemic stem cells (LSCs) driving Acute Myeloid Leukemia (AML). HI-STEM research has identified novel metabolic vulnerabilities of LSCs that next to metabolic pathways also regulate DNA methylation and also identified a super-enhancer (BENC) that controls the MYC oncogene. In another study a mechanism could be identified that allows LSCs to escape Natural-Killer (NK) cell mediated immune surveillance and the team identified a strategy using PARP1-inhibitors to re-establish NK recognition. These pre-clinical data are soon further evaluated in the NAKIP-AML clinical trial in which AML patients with minimal-residual disease after therapy are treated with PARP1-inhibitor followed by transfusion of haplo-identical NK-cells. These should now recognize and eliminate the residual therapy-resistant LSCs. Most recently, HI-STEM reported a new biomarker to identify patients that would/would-not respond to first-line therapy with Venetoclax (BCL-2 Inhibitor) / Azacytidine (hypomethylation agent). This is an important clinical advance as clinicians have two choices to treat fit AML patients at diagnosis: Combination chemotherapy or Venetoclax / Azacytidine. The biomarker measures the combinatorial expression of BCL-2 family member proteins specifically in LSCs from which a score (MAC-Score) is calculated that predicts response to Venetoclax/Azacytidine with high accuracy and may spare patients a highly toxic chemotherapy.
The main cause of death in patients with solid cancers are metastases. Cancer stem cells (CSCs) play an important role in metastasis and researchers of HI-STEM have identified and characterized blood circulating CSCs in breast cancer patients that have the capacity to initiate new metastasis. They discovered a connection between cellular stress and metastases and identified the Jun-kinase signalling pathway as one of the main stress switches of breast cancer CSCs. In pancreatic cancer HI-STEM identified an aggressive and metastatic subtype that differs in a specific methylation pattern that activates endogenous retroviruses leading to an inflammatory, but targetable tumor microenvironment. Finally, with overexpression of CYP3A5 a novel mechanism mediating therapy resistance to chemotherapy was identified and the insights are currently evaluated in a clinical trial (IntenSify) by treating patients with a CYP3A-inhibitor in combination with the chemotherapeutics paclitaxel. Next to a significant number of patents, researchers of HI-STEM have published >200 papers (since 2008) with last-author publications in highest ranked journals including Nature, Science, Cell, Cancer Discovery, Cell Stem Cell, Nature Cancer or Cancer Cell among others.
Links:
https://www.hi-stem.de
ProtaGene Continues the Legacy of GeneWerk's Commitment to Safer Gene and Cell Therapies

© ProtaGene GmbH
PPS and BioAnalytix were leading global contract research organizations primarily specializing in advanced analytical services for protein-based biopharmaceutical products. The combination of the three organizations established a world-class CRO designed to meet the evolving needs of today's biotherapeutic and cell & gene therapy sectors.
After the merger, ProtaGene maintained GeneWerk's site based in Heidelberg near the DKfZ, and serves as a center of excellence for Integration Site Analysis and safety-relevant assays in Cell and Gene Therapy, carrying on the legacy of its predecessor. Moreover, ProtaGene benefits from the continued contributions of key scientists from the founding team who remain with the company. In 2022, ProtaGene opened a brand-new, state-of-the-art laboratory in the U.S. in the Burlington Bio Center, located within suburban Boston's rapidly growing biotechnology hub. As a result, the cutting-edge technologies pioneered in Heidelberg are now poised to make a significant impact in the United States, the world's largest market for cell and gene therapy.
ProtaGene's services for the gene and cell therapy sectors include the valuable bioanalysis approaches Integration Site Analysis (ISA), biodistribution and vector shedding as well as numerous CMC offerings for supporting product development. GeneWerk innovations in vector analysis and quality control, off-target analysis of gene editing tools, immune repertoire analysis, and bioinformatic analysis of Next Generation Sequencing (NGS) data are an important part of ProtaGene’s offerings.The methods of these services were developed and adapted to these goals by founding GeneWerk researchers.
For example, one of the company's bioanalysis services, (nr)LAM-PCR-enabled ISA, was developed by one of GeneWerk's founders and became an FDA- and EMA-approved standard procedure for integrational gene therapies safety assessments. This method, together with a sophisticated bioinformatical analysis, was further matured over time for different vector applications, and nowadays requested broadly by biopharma organizations to understand the safety of their drug products.
During gene therapy development, the off-target integration of vectors in the genome may cause adverse effects, such as tumorigenesis. ISA is the key tool to ensure the biosafety of gene therapies by assessing integration profiles and allowing the clonal tracking of the modified cells in vivo. ProtaGene also provides off-target analyses of gene editing tools, such as CRISPR or TALENs, using LM-PCR and S-EPTS methods adapted specially for this task.
Additionally, ProtaGene offers various vector analytical and quality control services that align with FDA and EMA regulations. These analytic methods include the exact determination of the vector copy number and vector stability, the assessment of replication efficacy, and analysis of nucleic acid composition and post translational modifications.
Additionally to laboratory-based services, the company offers bioinformatical services to aid the analysis of NGS data. NGS is a parallel sequencing technology that provides a large-scale and rapid determination of the nucleotide sequence of whole genomes; however, the method generates vast amounts of data, of which analysis is complicated and requires expertise. ProtaGene's bioinformatics team can develop customized data analysis pipelines to test gene therapies.
