Division of Molecular Neurobiology
Prof. Dr. Ana Martin-Villalba
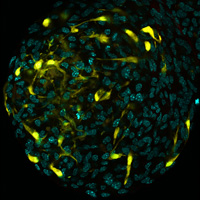
Neurosphere derived from neural stem cells from the subventricular zone of Nestin-Cre-ERT2 iYFP reporter mice 24 hours after tamoxifen induction.
© dkfz.de
Adult stem cells are defined by their ability to self renew and generate terminally differentiated cells of different lineages. These cells are therefore important for maintenance of tissue homeostasis and repair; aberrant regulation of these processes may result in tumor initiation and progression. Our laboratory is particularly interested in understanding stem cell heterogeneity and plasticity. How many differently committed stem cells do we have in the adult brain? How many different subtypes do we actually need for efficient brain repair? How restricted is stem cell plasticity in the brain compared to other adult organs? What are the molecular triggers and activators of plasticity and how are they linked to tissue repair and tumor initiation/progression? To tackle these questions we rely on state-of-the-art technologies that enables studying of these processes at different levels: (1) molecular: including the study of chromatin, transcription and translation regulation; (2) single cell: including clonal analysis and single cell “omics”; (3) organ: focusing on stem cell’s interaction with other local cell types and immune cells; and (4) organismal level: including genetic and disease animal models.
Unraveling the complex functions of CD95 in homeostasis and disease is the second major topic of the laboratory. In particular, we have discovered the multiple roles that CD95 plays in different neural, immune and tumor cells. We have identified CD95 as a modulator of survival, activation and lineage commitment in stem cells and inflammation. Aberrant regulation of CD95 activity crucially contributes to brain and pancreatic tumor progression. Identification of the different arms of action of CD95 should facilitate the device of targeted therapies against cancer, and inflammatory and neurodegenerative disorders.
FUTURE OUTLOOK
Our current and future research focuses on understanding the molecular program involved in the control of stem cell quiescence/ activation and plasticity, and the reversibility of these processes. Findings from adult stem cells will be further examined in human cancer stem cells and animal models of cancer. Our research aims at identifying molecular mechanisms involved in stem cell biology and tumor initiation and/or progression.

