Growth factor mediated alternative splicing of HPV oncogene expression
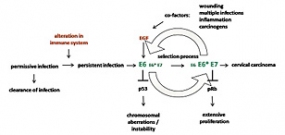
Model of HPV-induced carcinogenesis
© dkfz.de
Certain types of human papillomaviruses (HPVs) are etiologically linked to cervical cancer. Their transforming capacity is based on two main viral oncogenes E6 and E7, which are encoded by a polycistronic pre-mRNA. During carcinogenesis, the deregulated expression of the oncogenes is believed to initialize immortalization of the host cells, but for final malignant conversion additional co-factors are required. In a recent publication, Dr. Simone Rosenberger and colleagues in the group of Prof. Frank Rösl could show that the regulation of the oncogene expression by alternative splicing events was dependent on the epidermal growth factor (EGF). The presence of EGF was coupled to preferential E6 expression, whereas depletion of EGF or inhibition of the EGF-receptor resulted in E6 alternative exon exclusion, in favor of a non-functional truncated E6 variant termed E6*, accompanied by increased expression of the E7 protein. Changes in oncogene expression after EGF deprivation could consequently be verified by increased p53 levels and a reduction of the retinoblastoma protein pRb, two major cellular target proteins of E6 and E7. E6 exon exclusion upon EGF depletion was independent from promoter usage, mRNA stability, or selective mRNA transport within the cells. Time-course experiments and incubation with a translation inhibitor demonstrated that E6 alternative splicing is a direct and reversible effect of EGF signal transduction, not depending on de novo protein synthesis. Within this process, Erk1/2-kinase activation was the critical event for E6 exon inclusion, mediated by the upstream MAP kinase MEK1/2. Moreover, siRNA knockdown experiments revealed an involvement of splicing factors hnRNPA1 and hnRNPA2, Brm and Sam68 in this process. Because there is a natural gradient of EGF and EGF receptor expression in the stratified epithelium, EGF expression can be stimulated by various known risk-factors like wounding and inflammation. It is reasonable to assume that EGF influences the oncogene expression during the viral life cycle and during malignant transformation.
In the context of HPV-mediated immortalization and subsequent transformation (see schematic overview), it is tempting to speculate that enhanced E6 exon inclusion and in turn full-length E6 expression is first required for p53 labilization, thereby favoring chromosomal destabilization as initial early event in carcinogenesis. In a second selection step, when cells become more independent from external growth factors, preferential E6 exon exclusion and high E7 expression gain a selective growth advantage during progression, providing a consistent explanation for the prevalence of E6* mRNA found in most cervix carcinoma cell lines and CIN lesions.
Rosenberger S, De Castro-Arce J, Langbein L, Steenbergen RD, Rösl F. (2010). Alternative splicing of human papillomavirus type-16 E6/E6* early mRNA is coupled to EGF signaling via Erk1/2 activation. Proc. Natl. Acad. Sci. U S A., 107: 7006-7011.
www.ncbi.nlm.nih.gov/pubmed/20351270
