Radiooncology / Radiobiology
- Imaging and Radiooncology

Prof. Dr. med. Dr. h. c. Michael Baumann
Our research group bridges radiobiological research with clinical practice and consistently implements the translational research approach of “byte-to-bench-to-bedside” with the aim of further optimizing cancer therapies. At the same time, we also follow the opposite approach by using clinical observations to uncover and systematically analyse the underlying biological mechanisms of radiotherapy. Operative Division Head: Dr. Ina Kurth
Image: Spheroid,
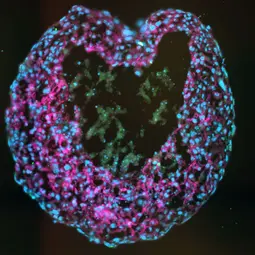
Image: Spheroid,
Our Research
The multidisciplinary work of our group, involving clinicians, biologists, bioinformaticians and physicists, is driving innovation from basic biology to clinical trials, advanced imaging techniques and data analyses in radiobiology and radiation oncology. A central point is to understand the biological heterogeneity of tumours. Despite the personalization of modern radiotherapy, this influences the response of patients to therapy, which leads to different reactions to therapy, resistance and relapses.
With the help of omics data and multiplex biomarkers from imaging, we want to refine the stratification of patients and develop novel, targeted (combination) treatments. Understanding the molecular basis of radioresistance and validating biomarkers in large patient cohorts are key to this.
In collaboration with different working groups from the DKFZ and the German Cancer Consortium (DKTK) and worldwide, our projects focus on:
Investigation of radioresistance mechanisms, including hypoxia and tumour heterogeneity.
Validation of biomarkers and testing of therapy combinations in preclinical models.
Integration of omics, pathology and imaging data for precision medicine.
Advancing preclinical and clinical studies via the RadPlanBio platform.
Theoretical modelling for analysis and interpretation of tumour evolution and survival datasets.
Introduction and development of standardized semantic structures to improve the usability of data across studies and institutions.
Outlook
Our research paves the way for clinical trials aimed at individually tailored personalized cancer treatments. Future therapies will shift from single-modality approaches to customized combinations that take into account the unique clinical and biological profile of each patient. By analysing the molecular mechanisms of radioresistance and tumour heterogeneity, we aim to identify key intracellular signalling pathways linked to treatment failure, revealing new druggable targets to enhance radiotherapy effectiveness.
Advanced multiplex histologic imaging parameters will be integrated with clinical and biological data to refine patient stratification to enable precise, effective and minimally invasive treatments with improved outcomes.
Projects
Within the department, there are - next to the subdivisions - various projects on which our research is focused.
1. Tumour Biology and Radiotherapy
HypoxRM - Imaging and Multiomics integration for tumour hypoxia characterization
This research project aims to enhance our understanding and improve therapeutic strategies for squamous cell carcinoma of the head and neck (HNSCC), a malignant tumour arising from various sites in the oral cavity and upper respiratory tract and, despite many treatment options, is still associated with a poor survival prognosis.
Radiation therapy remains a cornerstone of HNSCC treatment. However, therapeutic resistance frequently develops, partly due to hypoxia - regions of the tumour with insufficient oxygen supply. Strategies to sensitize hypoxic cancer cells to radiation, such as enhancing oxygen availability during treatment, have been explored. Interestingly, "reoxygenation" can also occur following radiation exposure, yet this process may paradoxically compromise treatment efficacy. This underscores the critical role of transiently hypoxic tumour regions in influencing patient responses to therapy.
It is now well-recognized that hypoxia is a highly dynamic phenomenon affecting virtually all cell types within the tumour microenvironment, presenting significant challenges for its accurate modelling in laboratory settings. Our primary objective is to bridge the gap between findings from in vitro and in vivo preclinical studies and the complex reality of hypoxia in cancer patients before and during treatment.
To achieve this, we are adopting innovative methodologies to assess oxygenation at the cellular level. Leveraging biobank resources (comprising collections of biological samples) and state-of-the-art imaging technologies, we aim to investigate individual cells and their microenvironment. Through this approach, we seek to elucidate how fluctuating oxygen levels influence cancer cell behaviour and treatment outcomes. Ultimately, our research aspires to pave the way for more effective therapeutic interventions for HNSCC in the presence of hypoxia.
Contact: Maria José Besso
Sex-related Differences in HNSCC Tumour Microenvironment
Our research investigates the intricate interplay between the tumour microenvironment (TME) and cancer progression in head and neck squamous cell carcinoma (HNSCC), with a particular focus on sex-specific differences. Leveraging multi-omics approaches, including single-cell transcriptomics and advanced imaging techniques, we aim to uncover how factors like sex chromosome dosage and HPV status influence the immune and stromal compartments of the TME. By integrating these diverse data layers, we strive to provide new insights into sex-specific dynamics in cancer, ultimately paving the way for more personalized and effective therapeutic strategies.
Contact: Cristina Conde Lopez
Tumour Heterogeneity and Radiosensitivity
This project focuses on understanding how tumour heterogeneity impacts radiosensitivity in head and neck squamous cell carcinoma (HNSCC). Tumour heterogeneity, both in molecular profiles and spatial organization, complicates the prediction of radiotherapy outcomes. We aim to link distinct tumour characteristics, including intratumoural heterogeneity, with radiosensitivity to identify predictive markers and improve therapeutic precision. By addressing tumour complexity, we hope to develop strategies that better account for heterogeneity and lead to more effective radiotherapy approaches for HNSCC patients.
Contact: Safayat Mahmud Khan
HPV and Radiosensitivity
In this project, we aim to explore the link between Human Papilloma Virus (HPV) 16 status and radiosensitivity in head and neck squamous cell carcinoma (HNSCC). While HPV-positive patients typically show better outcomes with radiotherapy compared to HPV-negative patients, a subset of HPV-positive cases exhibit poor prognosis. We are leveraging multi-omics data to uncover the underlying biological differences that drive this variability in radiosensitivity. Our goal is to identify key biomarkers and pathways associated with poor response in HPV-positive cases and use these insights to improve patient stratification and optimize radiotherapy outcomes.
Contact: Safayat Mahmud Khan
AXL as potential target for radiosensitization of head and neck squamous carcinoma (HNSCC)
AXL, a receptor tyrosine kinase, plays a critical role in tumour progression, metastasis, and resistance to therapies. AXL overexpression, in turn, can provide survival signals that mitigate the effects of DNA damage and apoptosis, indirectly complementing the loss of p53's tumour suppressive function.
In our HNSCC models AXL gene expression correlates with radioresistance.
We further investigate the association of the biological characteristics with radiosensitization and evaluate if there is a radiosensitizing effect in HNSCC by the AXL receptor activity.
Contact: Carla Popp
Clinical Cooperation Unit - Section Experimental and Translational Head and Neck Oncology
The main objectives of research program of the Section Experimental and Translational Head and Neck Oncology focus on cellular and molecular processes in the pathogenesis of head and neck cancer (HNC) as well as underlying principles of intrinsic and acquired treatment resistance and cancer cell dissemination. Of particular interest are gene regulatory networks and signalling pathways that modulate the mutual crosstalk between cancer cells and components of the tumour microenvironment, including the immune and nervous systems.
Our vision is a more effective and less toxic treatment of HNC patients by the establishment of predictive and prognostic risk models and the identification of vulnerabilities for molecularly stratified subgroups at a higher risk for treatment failure.
The experimental and analytical portfolio consists of prospective and retrospective cohort studies and biospecimens derived thereof, integrative analysis of multi-omics data utilizing well-established computational workflows and bioinformatics, establishment and analysis of pre-clinical models (e.g. mouse models and ex vivo cultures) as proof-of-concept studies for innovative therapeutic strategies, and 2D as well as 3D cell culture and co-culture models to address basic scientific concepts.
Contact: Jochen Heß
2. Data-Driven Cancer Research
Survival analysis of HNSCC through Histopathology Slides and AI
Together with our collaboration partner in Dresden (Division of Clinical Artificial Intelligence, TU Dresden, Prof. Jakob Kather), we are analysing which potential markers can be extracted from histology slides, particularly hematoxylin and eosin (H&E) stains. For this, foundation models are utilized as rich feature extractors of histopathological characteristics, in combination with attention-based models to weight areas of the images that are associated with survival. These models are either trained on specific molecular characteristics (e.g., omics modalities) or on available clinical data (e.g., follow-up information). With H&E stains being widely available in clinical practice, this method offers a cost-efficient way to identify prognostic features.
Contact: Verena Bitto
3. Theoretical Models in Cancer Research
Propagator Methods for Survival Analysis
In this project, inspired by the concepts and ideas of quantum mechanics, we aim to model the survival of patients and their tumour growth with a patient-near stochastic process. We imagine time-dependent patient data as positional states in an abstract space and try to describe a transition function (propagator) between these states; the parameters of this function can be understood as “natural laws of cancer growth”. In this way, we aim to characterise both deterministic and seemingly random aspects of disease progression and develop a novel method to extract information from survival data, in particular by incorporating temporal evolution. This represents a crucial step for scientific understanding and subsequent individualised clinical treatment.
Contact: Julian Schlecker
4. Data Integration and FAIRification
FAIRification of preclinical and clinical data
Our work focuses on standardizing and formalizing data collected from preclinical and clinical studies to enhance knowledge discovery. We began by standardizing digitized data hosted on the RadPlanBio platform, structuring it according to the SEND standard for nonclinical datasets. The data is then formalized using the Ontology for Preclinical Trials in Radiation Oncology (PTRO). Building on this foundation, we are using Ontop to develop a Virtual Knowledge Graph (VKG) based on the PTRO ontology. This VKG allows users to access and query heterogeneous data sources as a unified graph, without physically moving or duplicating the data. These tools provide a structured and transparent representation of data, ensuring clarity, consistency, and reproducibility across studies. Looking ahead, we are extending these FAIRification efforts to clinical data, paving the way for greater integration, accessibility, and usability across the research continuum.
Contact: Olga Giraldo Pasmin
Team
Our highly interdisciplinary division consists of scientists, technical and documentary assistants as well as students who have received their training in the fields of biology, physics, medicine, bioinformatics and computer science. Experienced scientists work side by side with PhD/MD students and undergraduates to advance our research within the group and in global collaborations.
-

Prof. Dr. med. Dr. h. c. Michael Baumann
Division Head
- Show profile

Dr. Ina Kurth
Division Head
-
Silke Cardona
Secretary Research Topic Imaging and Radiooncology
-

Jana Bensing
Medical Documentation Assistance
- Show profile

Dr. Maria José Besso
Biotechnologist, Hypoxia and Radioresponsiveness in HNSCC
- Show profile

Dr. Verena Bitto
Computational Scientist
- Show profile

Mahnaz Bonrouhi
Biological-technical Assistant
- Show profile

Cristina Conde Lopez
Sex differences in cancer
- Show profile
Manuela Dittrich
Biologist
- Show profile

Rosemarie Euler-Lange
Bioengineer
-

Thomas Früchtel
Medical Documentation
- Show profile

Dr. Olga Ximena Giraldo Pasmin
Ontology Engineer
- Show profile

Dr. Wahyu Wijaya Hadiwikarta
Head of Knowledge Modelling & RadPlanBio
- Show profile

Priv. Doz. Dr. Jochen Heß
Clinical Cooperationpartner for Experimental and Translational Head- and Neck-Oncology
- Show profile

Safayat Mahmud Khan
HPV, Radiosensitivity and Tumor Heterogeneity
- Show profile

Carla Popp
AXL and Radiosensitivity
- Show profile

Dr. Mareike Roscher
Head of Morszeck PCTU
-

Joana Schlag
Medical Documentation Assistance
- Show profile

Julian Schlecker
Survival Models
-

Büsranur Yilmaz
Medical Documentation Assistance
Selected Publications
Besso MJ, Bitto V, Koi L, Wijaya Hadiwikarta W, Conde-Lopez C, Euler-Lange R, Bonrouhi M, Schneider K, Linge A, Krause M, Baumann M, Kurth I.
Heidelberg Institute for Radiation Oncology (HIRO)
Our division is member of the Heidelberg Institute for Radiation Oncology (HIRO).

Get in touch with us

Dr. Ina Kurth
Division Head