RadPlanBio as-a-Sevice

Dr. Wahyu Wijaya Hadiwikarta
Head of Knowledge Modelling & RadPlanBio
RadPlanBio is a dose-space-time-resolved database platform for preclinical and clinical translational research in radiation oncology and radiobiology. As a dedicated operating site, we provide a scalable, service-oriented infrastructure for managing and analysing study data. Our platform enables acquisition, curation, tabulation and analytical presentation to ensure high-quality, reproducible research.

Background
The investigation and validation of prognostic factors are crucial in cancer research to optimize treatment strategies. Clinical and preclinical studies generate datasets for identifying and predicting these factors, forming the foundation of evidence-based clinical pathways that ultimately guide patient care. Reliable outcomes depend on accurate and standardized data management to ensure analytical validity. RadPlanBio was developed to address these critical data requirements.
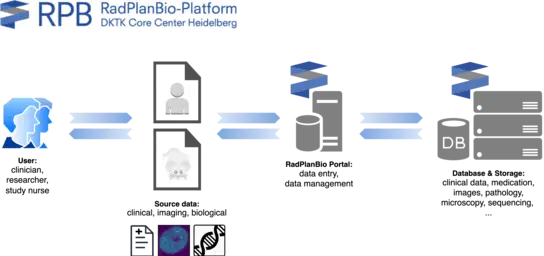
RadPlanBio is a robust and scalable IT platform for large-scale, multi-center data collection, analysis, and quality assurance in radiation oncology research, both within the German Cancer Consortium (DKTK) and beyond. It plays a central role in fostering collaboration among DKTK centers, ensuring efficient data integration and supporting high-quality, reproducible research in radiooncology and radiobiology.
Our Service
RadPlanBio is a modular application suite that integrates open-source components for the hypothesis-driven design of preclinical and clinical studies. It includes:
- Electronic Data Capture (EDC) system for secure data capture via electronic Case Report Forms (eCRFs).
- Picture Archiving and Communication System (PACS) for efficient DICOM data management.
- Data Warehouse for the storage of extensive object data (e.g. sequencing data, microscopy images).
Essential functions:
- Role-based access control for data entry, monitoring and analysis, with site-specific restrictions.
- Compliance with Good Clinical Practice (GCP) and CDISC standards.
- Pseudonymization / anonymization of patient data.
RadPlanBio supports the design of preclinical and clinical studies and integrates a time-event model to monitor the progress of the study in a structured way.
Contact
If you are planning a preclinical or clinical study and are interested in using RadPlanBio for your data management solution, please contact us at: radplanbio(at)dkfz-heidelberg.de
Selected References
Hadiwikarta W., Ebert N., Roscher M., Kurth I., Baumann M.
Skripcak T., Just U., Simon M., Büttner D., Lühr A., Baumann M., Krause M.
Skripcak T., Belka C., Bosch W., Brink C., Brunner T., Budach V., Büttner D., Debus J., Dekker A., Graui C., Gulliford S., Hurkmans C., Just U., Krause M., Lambin P., Langendijk J.A., Lewensohn R., Lühr A., Maingon P., Masucci M., Niyazi M., Poortmans P., Simon M., Schmidberger H., Spezi E., Stuschke M., Valentini V., Verheij M., Whitfield G., Zackrisson B., Zips D., Baumann M.
Get in touch with us

Dr. Wahyu Wijaya Hadiwikarta
Head of Knowledge Modelling & RadPlanBio