PROBASE - Study
In the PROBASE study (Risk-adapted prostate cancer early detection study based on a ‘baseline’ PSA value in young men - a prospective multicenter randomised trial), a modern concept for early prostate cancer detection is being investigated. In this risk-adapted strategy, measurements of the prostate-specific antigen (PSA) in the blood are carried out depending on the individual risk of the man, which is determined using a baseline PSA value at the age of 45 or 50.

Study meetings

“Our aim is to reduce the number of examinations and at the same time to keep a closer eye on men at increased risk by monitoring them more closely.”
Peter Albers, director PROBASE-Study
General information
The determination of PSA levels in the blood plays an important role in the diagnosis of prostate cancer - known as prostate carcinoma by doctors - as well as in the monitoring of prostate cancer therapy. In early detection, however, it is controversial. On the one hand, PSA screening, i.e. the determination of PSA levels in all men over a certain age at regular intervals, allows prostate cancer to be detected earlier and therefore treated more effectively, which reduces mortality.
On the other hand, men with prostate cancer sometimes have such a favorable prognosis that they do not necessarily need treatment. However, this often begins when prostate cancer is detected. In addition, PSA measurements can produce results that falsely indicate the presence of prostate cancer. For this reason, general PSA screening can lead to further, often physically and psychologically stressful examinations and treatments that would not have taken place without screening.
Against this background, men aged 45 and over were entitled to reimbursement for an annual palpation examination under the statutory health insurance system until 2025. However, PSA testing has only been reimbursed if the man has symptoms that indicate prostate disease. Men who wanted early detection through PSA testing had to pay for this and, if necessary, an ultrasound examination of the prostate themselves as an individual health service (IGeL).
In the PROBASE study (Risk-adapted prostate cancer early detection study based on a ‘baseline’ PSA value in young men - a prospective multicenter randomised trial), which was initiated by Prof. Peter Albers and epidemiologist Prof. Nikolaus Becker, a modern concept for early prostate cancer detection is being investigated. In this risk-adapted strategy, measurements of PSA in the blood are carried out depending on the individual risk of the man, which is determined using a baseline PSA value at the age of 45 or 50.
The PROBASE study is led by the German Cancer Research Center and funded by German Cancer Aid.

The patron of the study is Wolfgang Bosbach.
News and initial results from the PROBASE study

- PROBASE study as basis for new prostate cancer guideline (only german available)
- Palpation examination not suitable for early detection of prostate cancer in 45-year-old men - BEST PAPER AWARD European Urology Oncology 2023
- Low risk usually remains low
Study information:
Revised PROBASE study information version 5.1 general (2023) (PDF | 318,21 KB)
Articel in the journal of the German Research Centre “einblick” 01/2023:
“The individual risk” (only german version available)
Television report on NDR
On 30.01.2024, the NDR television program "Visite" ran a report entitled "Prostatakrebs: Ursachen, Symptome, Behandlung". The PROBASE Study Center Hanover took part in the filming. The video of the report is available in the NDR media library (only german version).
Study program
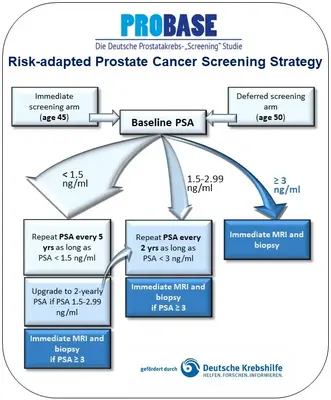
In the PROBASE study, four study centers (Düsseldorf University Hospital, Hannover Medical School, Heidelberg University Hospital, Klinikum rechts der Isar of the Technical University of Munich) recruited more than 46,000 men aged 45 throughout Germany between 2014 and 2019, who were invited to participate in the study via the residents' registration offices.The participants were randomly divided into two groups in a 1:1 ratio (independent participation was not possible because this is an epidemiological study, which would otherwise be biased in the selection of participants):
- Group A received the first PSA test at the age of 45
- Group B received the first PSA test at the age of 50
The subsequent risk-adapted PSA screening is identical in both groups:
For men with a baseline PS A level below 1.5 ng/ml, further PSA tests are performed at five-year intervals.
For men with a baseline PSA value of 1.5-2.99 ng/ml, who have a higher risk of developing the disease, further PSA measurements are carried out every two years.
As soon as the PSA value is 3 ng/ml or higher - at the beginning or in the follow-up tests - further examinations follow.
The study ends for all participants at the age of 60. Around 90% belong to the low-risk group, in which PSA tests are carried out at five-year intervals, so that four PSA tests up to the age of 60 will be sufficient if measurements begin at the age of 45.
Contact us
Prof. Dr. Peter Albers
Tel.: (0211) 81 08 239
probase(at)med.uni-duesseldorf.de
Prof. Dr. Jan Phillip Radtke
Jale Lakes
Tel.: (0211) 81 08 239
probase(at)med.uni-duesseldorf.de
Urological University Clinic Düsseldorf
Moorenstr. 5
40225 Düsseldorf
Prof. Dr. Markus Kuczyk
PD Dr. Nina Harke
Clinic for Urology and Urological Oncology
Hannover Medical School
Carl-Neuberg-Straße 1
30625 Hannover
Hanover Study Center
Tel.: (0511) 532 48 92
probase(at)mh-hannover.de
Prof. Dr. Dr. Jürgen Debus
Dr. Christoph Grott
Department of Radiation Oncology and Radiotherapy
Heidelberg University Hospital
Im Neuenheimer Feld 400
69120 Heidelberg
Heidelberg Study Center (New address!):
Tel.: (06221) 42 5488
probase(at)med.uni-heidelberg.de
DKFZ National Cancer Prevention Center: Berliner Straße 45, Prevention Outpatient Clinic 1st floor
Prof. Dr. Jürgen Gschwend
Tel.: (089) 414 098 22
probase.uro(at)mh.tum.de
Prof. Dr. Kathleen Herkommer
Tel.: (089) 414 098 22
probase.uro(at)mh.tum.de
Clinic and Polyclinic for Urology at the Technical University of Munich
Ismaninger Straße 22
81675 Munich
Study administation and reference institutions
Prof. Dr. Peter Albers
Department of Personalized Early Detection of Prostate Cancer
Phone: 06221 42 3046
E-mail: p.albers(at)dkfz.de
Prof. Dr. Rudolf Kaaks
Department of Cancer Epidemiology
Tel.: 06221 42 2200
E-mail: r.kaaks(at)dkfz.de
Dr. Petra Seibold
Phone: 06221 422208
E-mail: p.seibold(at)dkfz.de
Reference Pathology
Institute of Pathology
University Hospital Bonn
Prof. Dr. Glen Kristiansen
Reference Radiology
Institute for Diagnostic and Interventional Radiology
Düsseldorf University Hospital
Prof. Dr. Gerald Antoch
Reference Nuclear Medicine
Clinic for Nuclear Medicine
Düsseldorf University Hospital
Prof. Dr. Frederik Giesel
Selected Publications
Krilaviciute, A.; Kaaks, R.; Seibold, P.; De Vrieze, M.; Lakes, J.; Radtke, J. P.; Kuczyk, M.; Harke, N. N.; Debus, J.; Fink, C. A.; Herkommer, K.; Gschwend, J. E.; Meissner, V. H.; Benner, A.; Kristiansen, G.; Hadaschik, B.; Arsov, C.; Schimmöller, L.; Antoch, G.; Giesel, F. L.; Makowski, M.; Wacker, F.; Schlemmer, H. P.; Becker, N.; Albers, P.
Boschheidgen, M.; Albers, P.; Schlemmer, H. P.; Hellms, S.; Bonekamp, D.; Sauter, A.; Hadaschik, B.; Krilaviciute, A.; Radtke, J. P.; Seibold, P.; Lakes, J.; Arsov, C.; Gschwend, J. E.; Herkommer, K.; Makowski, M.; Kuczyk, M.; Wacker, F.; Harke, N. N.; Debus, J.; Koerber, S. A.; Benner, A.; Kristiansen, G.; Giesel, F. L.; Antoch, G.; Kaaks, R.; Becker, N.; Schimmöller, L.
Krilaviciute, A.; Becker, N.; Lakes, J.; Radtke, J. P.; Kuczyk, M.; Peters, I.; Harke, N. N.; Debus, J.; Koerber, S. A.; Herkommer, K.; Gschwend, J. E.; Meissner, V. H.; Benner, A.; Seibold, P.; Kristiansen, G.; Hadaschik, B.; Arsov, C.; Schimmöller, L.; Giesel, F. L.; Antoch, G.; Makowski, M.; Wacker, F.; Schlemmer, H. P.; Kaaks, R.; Albers, P.
Krilaviciute, A.; Albers, P.; Lakes, J.; Radtke, J. P.; Herkommer, K.; Gschwend, J. E.; Peters, I.; Kuczyk, M.; Koerber, S. A.; Debus, J.; Kristiansen, G.; Schimmöller, L.; Antoch, G.; Makowski, M.; Wacker, F.; Schlemmer, H. P.; Benner, A.; Giesel, F. L.; Siener, R.; Arsov, C.; Hadaschik, B.; Becker, N.; Kaaks, R.







