Targeted Tumor Vaccines
Our aim is to develop strategies that might be useful in adoptive cell therapy (ACT) of solid tumors. One such strategy developed recently by our research group is the combination, in the same effector cell, of a TCR and a chimeric antigen receptor (CAR), each targeting a different antigen. This approach could be applied to enhance the efficacy of TCR-based cancer immunotherapy and to overcome limitations in the efficacy of ACT due to intratumoral antigen heterogeneity.

Our Research
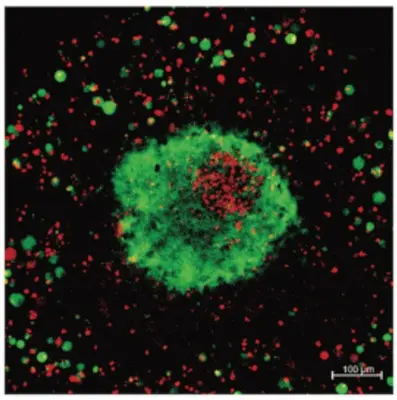
We are interested in identifying T cell receptors (TCRs) that recognize specific tumor antigens presented by the major histocompatibility complex class I (HLA) on the surface of tumor cells. Immune cells engineered to have an anti-tumor TCR may be effective in the treatment of eventually any type of cancer. However, because of some degree of promiscuity in antigen recognition, TCR-based immunotherapy may cause adverse effects due to engagement with one or more antigen(s) in healthy tissues, which may have homology with the target tumor antigen. To minimize such on-target off-tumor effect, we have developed a dual-receptor strategy consisting in combining a weak TCR, selected from the T cell repertoire of healthy donors, with a weak chimeric antigen receptor (CAR), understanding “weak” as low-avidity receptors. We have been able to show that NK and T cells armed with such dual receptors are highly cytotoxic against cancer cell lines expressing the antigens recognized by the respective receptors, while NK and T cells armed with only one of the receptors exhibited low cytotoxic activity against the same tumor cells and no activity against cells not expressing the target antigens.
Projects
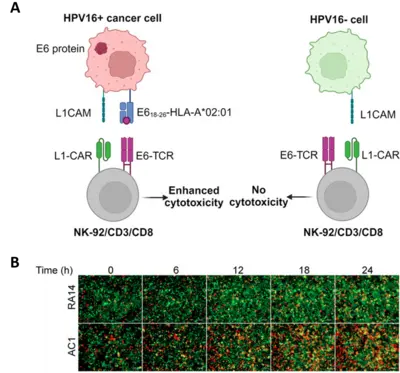
Dual TCR/CAR NK and T cells
The human papillomavirus type 16 (HPV16) causes a large fraction of genital and oropharyngeal carcinomas. To maintain the transformed state, the tumor cells must continuously synthesize the E6 and E7 viral oncoproteins, which makes them tumor-specific antigens. Indeed, specific T cell responses against E6 and E7 have been well documented and CD8+ T cells engineered to express T cell receptors (TCRs) that recognize epitopes of E6 or E7 have been tested in clinical studies with promising results, yet with limited clinical success. Using CD8+ T cells from peripheral blood of healthy donors, we have identified two novel TCRs reactive to an unexplored E618-26 epitope. These TCRs showed limited standalone cytotoxicity against E618-26-HLA-A*02:01-presenting tumor cells. However, a single-signaling domain chimeric antigen receptor (ssdCAR) targeting L1CAM, a cell adhesion protein frequently overexpressed in HPV16-induced cancer, prompted a synergistic effect that significantly enhanced the cytotoxic capacity of NK-92/CD3/CD8 cells armored with both TCR and ssdCAR when both receptors simultaneously engaged their respective targets, as shown by live microscopy of 2-D and 3-D co-cultures. Thus, virus-specific TCRs from the CD8+ T cell repertoire of healthy donors can be combined with a suitable ssdCAR to enhance the cytotoxic capacity of the effector cells and, indirectly, their specificity.
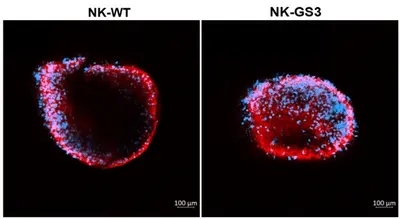
Heparanase (HPSE) enhances immune cell infiltration of tumors
Eradication of cancer cells by the immune system requires extravasation, infiltration and progression of immune cells through the tumor extracellular matrix (ECM). These are also critical determinants for successful adoptive cell immunotherapy of solid tumors. Together with structural proteins, such as collagens and fibronectin, heparan sulfate (HS) proteoglycans are major components of the ECM. Heparanase 1 (HPSE) is the only enzyme known to have endoglycosidase activity that degrades HS. HPSE is expressed at high levels in almost all hematopoietic cells, which suggests that it plays a relevant role in immune cell migration through solid tissues. Besides, tumor cells express also HPSE as a way to facilitate tumor cell resettlement and metastasis. Therefore, an increase in HPSE in the tumor ECM would be detrimental. Here, we analyzed the effects of constitutive expression of an active, membrane-bound HPSE on the ability of human natural killer (NK) cells to infiltrate tumors and eliminate tumor cells. We demonstrate that NK cells expressing a chimeric active form of HPSE on the cell surface as an integral membrane protein, display significantly enhanced infiltration capability into spheroids of various cancer cell lines, as well as into xenograft tumors in immunodeficient mice. As a result, tumor growth was significantly suppressed without causing noticeable side effects. Altogether, our results suggest that a constitutively expressed active HSPE on the surface of immune effector cells enhances their capability to access and eliminate tumor cells. This strategy opens new possibilities for improving adoptive immune treatments using NK cells.
Team
Selected Publications
Liborio-Ramos S, Quiros-Fernandez I, Ilan N, Soboh, S., Farhoud M, Süleymanoglu R, Bennek M, Calleja-Vara S, Müller M, Vlodavsky I and Cid-Arregui, A
Quiros-Fernandez, I, Liborio-Ramos, S, Leifert, L, Schönfelder, B, Vlodavsky, I and Cid-Arregui, A
Poorebrahim, M, Quiros-Fernandez, I, Marmé, F, Burdach, SEG, Cid-Arregui, A