Tumorvirus-specific Vaccination Strategies
- Immunology, Infection and Cancer

Prof. Dr. Martin Müller
The N-terminal region of the human papillomavirus (HPV) L2 protein has been shown to contain epitopes able to induce the production of neutralizing and cross-neutralizing antibodies. Using bacterial thioredoxin as a scaffold, we managed to significantly enhance the immunogenicity of putative L2 neutralizing epitopes. In the past years, we extensively optimized the prophylactic vaccine antigen and also determined its safety in an animal model.

Our Research
We now entered into a clinical development phase and are starting a phase I clinical trial (EudraCT No.: 2021-002584-22). This trial should demonstrate safety and immunogenicity. Our vaccine is based on a single molecule, is highly thermostable and more importantly, induces protective responses against all oncogenic HPV as well as a number of so called ‘low risk’ HPV. Therefore, the vaccine has the potential to provide protection against HPV also in regions where the current HPV vaccines cannot be distributed, which applies to about two thirds of all countries.
Jointly with the European Molecular Biology Laboratory (EMBL) in Heidelberg we have developed a standardized validated assay system which allows us the high-throughput detection of neutralizing antibodies against HPV16, HPV18, and other oncogenic HPV types (automated pseudovirion-based HPV-neutralization assay). This assay can be used for a multitude of epidemiological studies. In particular, the duration of the immunization-protection after the introduction of a new vaccine could be monitored with our assay in large study groups.
Current projects of our group
Yueru Zhang
The minor capsid protein L2 plays a critical role in virus assembly, infection, and replication. Although there have been some efforts to develop vaccines targeting the highly conserved immunogenic region within the L2 sequence, its structural properties remain largely unexplored. This lack of structural insight is primarily due to the inherent flexibility and instability of the full L2 protein. In this project, we aim to discover the structure of the immunogenic region of L2 by co-crystallizing it with corresponding neutralizing antibodies. By leveraging this structural and functional information, we hope to provide new perspectives on HPV vaccine development and optimization.
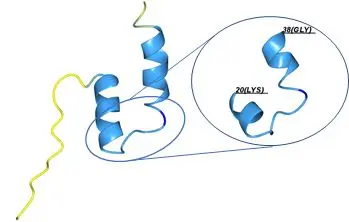
Predicted structure of HPV16 amino acids 1-45 was generated using AlphaFold 3. The immunogenic region is highlighted in the right panel. The structure is color-coded based on the prediction confidence (pLDDT): yellow indicates a pLDDT value between 50 and 70, light blue represents a pLDDT between 70 and 90, and blue indicates a pLDDT greater than 90.

Ecem Kaplan
HPV VLPs and capsomers have the potential to be used as a vaccine delivery platform due to their highly organized and repetitive structure. Due to its size, insertion of Trx-8mer epitope to surface exposed loops of L1 cannot be achieved without interfering with capsomer or VLP assembly. DogTag/DogCatcher was employed as a promising approach to decorate HPV 16 L1 capsomers or VLPs with L2 epitopes.

Heiko Weyd
Key aspect of our vaccine concept are the broadly protective humoral responses induced by the L2-based polytope antigen PANHPVAX, currently evaluated in a clinical phase I study. We have demonstrated that vaccine-induced protection is driven by anti-L2 neutralizing antibodies. However, comprehension on memory B cell and innate immune responses induced by L2-based vaccines is poor. In this project, we follow-up on our prior observations by analyzing PANHPVAX-induced immunity with respect to L2-specific non-neutralizing antibodies and antibody profile as well as L2-specific Fc-effector functions, namely antibody-mediated cellular cytotoxicity and phagocytosis.
Papillomaviruses induce benign and malignant tumors
Papillomviruses infect humans and many vertebrates. These infections can be completely asymptomatic but they could also result in skin warts or genital warts. Under certain circumstances infections with papillomaviruses can lead to the development of cancer. One major aim of vaccine development is to provide protection against infections by papillomaviruses to prevent development of cancer.
Prophylactic vaccines
So called prophylactic vaccines induce antibodies which are proteins of the immune system. These antibodies recognize and bind to virus particles that enter the body. This hinders entry of the viruses into the cells and thus the infection.
The vaccine: Virus-like particles -VLPs-
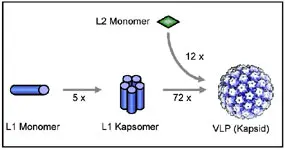
Papillomaviruses cannot be produced in sufficient quantities in cell culture. For a long time, this has prevented the development of a vaccine, e.g. based on inactivated viruses.

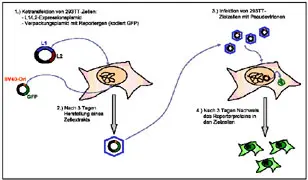
Besides the development of papillomavirus vaccine strategies we are interested to study the functions of the structural proteins L1 and L2 in the course of the viral life cycle. With the help of VLPs we are able to investigate interactions of the viruses with the cellular receptor. The L2 protein plays a central role in the packaging of the viral genome into the capsids. By packaging heterologous DNA into capsids we are able to produce so called pseudovirions. With these we can detect virus-neutralizing antibodies in the sera of immunized animals but also in human sera. In addition, pseudovirions allow to study the host range of the papillomaviruses.
Team
-
-
-
Luisa Fernanda Bustamante Jaramillo
-
Lea Herzel
-
Yllka Kabashi
-
Dr. Ecem Kaplan
-
-
Laura Saverino
-
-
Selected Publications
Filipe Mariz, Kathrin Balz, Manuela Dittrich, Yueru Zhang, Fan Yang, Xueer Zhao, Angelo Bolchi, Simone Ottonello, and Martin Müller
Mariz FC, Gray P, Bender N, Eriksson T, Kann H, Apter D, Paavonen J, Pajunen E, Prager KM, Sehr P, Surcel HM, Waterboer T, Müller M, Pawlita M, Lehtinen M
Zhao X, Yang F, Mariz F, Osen W, Bolchi A, Ottonello S, Müller M
Filipe Colaço Mariz, Noemi Bender, Devasena Anantharaman, Partha Basu, Neerja Bhatla, Madhavan Radhakrisna Pillai, Priya R Prabhu, Rengaswamy Sankaranarayanan Tiina Eriksson, Michael Pawlita, Kristina Prager, Peter Sehr, Tim Waterboer, Martin Müller, Matti Lehtinen
Get in touch with us



