Virotherapy
- Immunology, Infection and Cancer
- NCT
- Clinical Cooperation Unit

Prof. Dr. Dr. Guy Ungerechts
Head of Division
We explore new strategies for engineering effective oncolytic viruses and combination therapies, establish processes for manufacturing oncolytic virus formulations, perform early and late phase clinical virotherapy studies and decipher mechanisms of oncolytic immunotherapy in preclinical models and patients.
Our Research
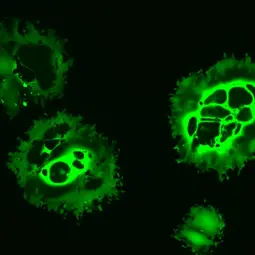
Virotherapy - Developing a unique type of cancer immunotherapy
Clinical observations of cancer remissions after viral infections laid the foundation for the field of virotherapy. Certain viruses are being explored or genetically engineered for selective replication in cancer cells, leading to tumor cell lysis. In recent years, it has become increasingly appreciated that these so-called oncolytic viruses act as a cancer immuno- (viro-) therapy via tumor vaccination effects in particular. In 2015, a first oncolytic virus was approved for the treatment of advanced melanoma in the US and Europe. Several other oncolytic viruses are currently being developed.
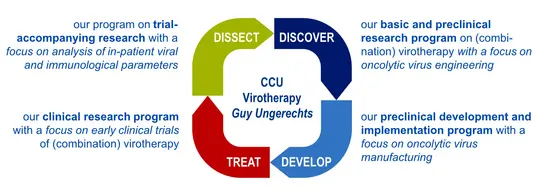
The CCU Virotherapy research agenda
We explore new strategies for engineering effective oncolytic viruses and combination therapies, establish processes for manufacturing oncolytic virus formulations, perform early and late phase clinical virotherapy studies and decipher mechanisms of oncolytic immunotherapy in preclinical models and patients (see here for an overview).
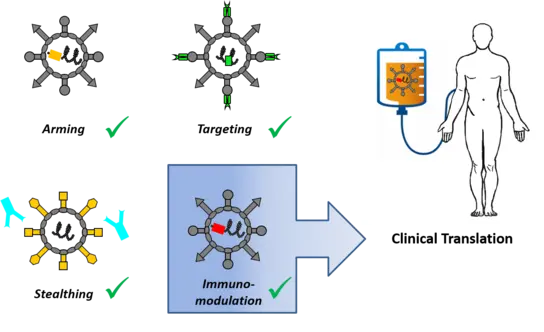
Engineering oncolytic agents for maximum anti-tumor efficacy and safety
Our preclinical research program focuses on a measles vaccine virus platform with more recent projects exploring oncolytic adenoviruses and parvoviruses. Using molecular cloning, we engineer oncolytic viruses and explore combination regimens for specific medicinal purposes (see “discovery projects”). For instance, we have designed viruses to
- direct viral infection to tumors by selective tumor cell entry or post-entry replication control (“targeting”)
- express therapeutic proteins to implement increased potency (“arming”). Payloads include cytokines, immune checkpoint inhibitors and bispecific antibodies for alerting the patient’s immune system to the tumor (“immuno-virotherapy”)
- establish effective combination treatments (e.g. “radio-therapy”)
Deciphering mechanisms of immuno-virotherapy
We develop and apply preclinical and patient-derived tumor models and systems immunodiagnostic tools to decipher factors both within tumor cells and the tumor microenvironment that determine oncolytic potency and anti-tumor immune activation. Our research aims at rational strategies for improving oncolytic viruses and identifying potential biomarkers of immuno-virotherapy for clinical translation (see “discovery projects” and “dissect projects”).
Advancing oncolytic viruses into clinical application
With our research, we constantly aim to identify the most effective immuno-virotherapies for clinical application. Based on our preclinical findings, we are preparing a phase I/II clinical trial with an oncolytic measles virus for immuno-virotherapy of advanced gastrointestinal cancers. To this end, we currently establish GMP-compliant virus manufacturing processes (see “develop projects”). Further completed, ongoing and upcoming trials (phases I - III) explored or explore, e.g. oncolytic parvoviruses, herpes and vaccinia viruses (see “treat projects”). Importantly, the trials we initiate are accompanied by translational research programs to pinpoint mechanisms of action and identify predictive biomarker signatures of successful immuno-virotherapy (see “dissect projects”).
Our Projects
Research Projects at the Clinical Cooperation Unit Virotherapy explore new strategies for engineering effective oncolytic viruses and combination therapies with programs along the translational research cycle from the lab to early clinical trials and back:
Team

Head of Division
-
-

Natalie Jäger
Secretary
Principal Investigators
Scientists
-

Dr. Johannes Heidbüchel
Scientist
-

Dr. Jonny Hertzog
Scientist
-

Dr. Eleonora Prodi
Staff scientist
-

Dr. Thomas Walle
Scientist
Technicians
PhD and MD students
-

Anmol Aggarwal
Doctoral Student
-
-
-
-
-
-
-
-
-
-
Selected Publications
Walle T, Bajaj S, Kraske JA, Rösner T, Cussigh CS, Kälber KA, Müller LJ, Boyoung Strobel S, Burghaus J, Kallenberger S, Stein-Thöringer C, Jenzer M, Schubert A, Kahle S, Williams A, Hoyler B, Zielske L, Skatula R, Sawall S, Leber MF, Kunes RZ, Krisam J, Fremd C, Schneeweiss A, Krauss J, Apostolidis L, Berger AK, Haag GM, Zschäbitz S, Halama N, Springfeld C, Kirsten R, Hassel JC, Jäger D, NCT ANTICIPATE Investigators & Ungerechts G
Hajda J, Leuchs B, Angelova AL, Frehtman V, Rommelaere J, Mertens M, Pilz M, Kieser M, Krebs O, Dahm M, Huber B, Engeland CE, Mavratzas A, Hohmann N, Schreiber J, Jäger D, Halama N, Sedlaczek O, Gaida MM, Daniel V, Springfeld C, Ungerechts G
Speck T, Heidbuechel JPW, Veinalde R, Jaeger D, von Kalle C, Ball CR, Ungerechts G, Engeland CE
Veinalde R, Grossardt C, Hartmann L, Bourgeois-Daigneault MC, Bell JC, Jäger D, von Kalle C, Ungerechts G, Engeland CE
Get in touch with us























