Malignant tumors spread throughout the body by releasing cancer cells into the bloodstream, which can reach distant organs and metastasize there. The success of a tumor cell circulating in the bloodstream to grow into a metastasis is highly dependent on the characteristics of the local environment. This process is often described as a 'seed' (circulating tumor cell) that must fall on fertile 'soil' (metastatic niche) for a new tumor to develop at the distant site. This concept leads to the hypothesis that an unsuitable 'soil' may help limit metastatic growth by allowing the scattered cancer cells to be kept in a dormant, state and not divide further.
But until now, little was known about how the microenvironment of a seeded tumor cell - known as the metastatic niche - evolves as the metastasis grows. To answer this question, a team of researchers from Heidelberg and Mannheim, led by Hellmut Augustin, together with colleagues from University College London, studied mice whose primary tumors were surgically removed. In this way, the scientists were able to observe the development of metastases in the absence of the primary tumor. “This intervention enabled us to distinguish for the first time which properties of the metastatic niche are controlled by the distant primary tumor and which are regulated locally,“ explains Hellmut Augustin, adding, “It is also essential that we were thus able to recreate the situation of tumor patients after surgery in the experimental system.“
With a particular focus on the lung, the researchers performed global gene expression analyses of the metastatic niche. They discovered that in the presence of a primary tumor, the endothelial cells lining the interior of blood vessels produced the protein LRG1 (leucine-rich alpha-2-glycoprotein 1) in large quantities. “The blood vessels produced LRG1 exclusively in the presence of the primary tumor, which stimulates the growth of nearby connective tissue cells in the lung. This creates a tumor cell growth-promoting microenvironment ('niche') where circulating tumor cells can settle and grow into lung metastasis,“ said DKFZ researcher Mahak Singhal, first author of the current study, adding, “This is the first time we have demonstrated that the metastasis-promoting effect of the niche is triggered over long distances by the primary tumor.“ From a certain size, the metastases then act like a primary tumor themselves, again promoting the formation of LRG1.
How does the progression of metastasis change when the key molecule LRG1 is blocked with an antibody? In fact, scientists were able to slow the metastatic growth of breast and lung tumors in this way. One of the most surprising findings of the current study was that LRG1 was not only produced by blood vessels at the metastasis site, but that it was produced by endothelial cells throughout the body and released into the circulation. Thus, the researchers were even able to detect the metastasis-promoting molecule directly in blood samples. “On the one hand, we can now detect LRG1 produced by endothelial cells as a biomarker indicative of a metastatic tumor. In addition, we want to validate LRG1 as a target for new therapeutic approaches that may be able to halt the metastatic spread of tumors,“ says study leader Hellmut Augustin, summarizing the current results.
Mahak Singhal, Nicolas Gengenbacher, Ashik Ahmed Abdul Pari, Miki Kamiyama, Ling Hai, Bianca J. Kuhn, David M. Kallenberg, Shubhada R. Kulkarni, Carlotta Camilli, Stephanie F. Preuß, Barbara Leuchs, Carolin Mogler, Elisa Espinet, Eva Besemfelder,Danijela Heide, Mathias Heikenwalder, Martin R. Sprick, Andreas Trumpp, Jeroen Krijgsveld, Matthias Schlesner, Junhao Hu, Stephen E. Moss, John Greenwood, Hellmut G. Augustin: Temporal multi-omics identifies LRG1 as a vascular niche instructor of metastasis.
Science Translational Medicine 2021, DOI: https://www.science.org/doi/10.1126/scitranslmed.abe6805
A picture is available for download:
Singhal_STM.jpg
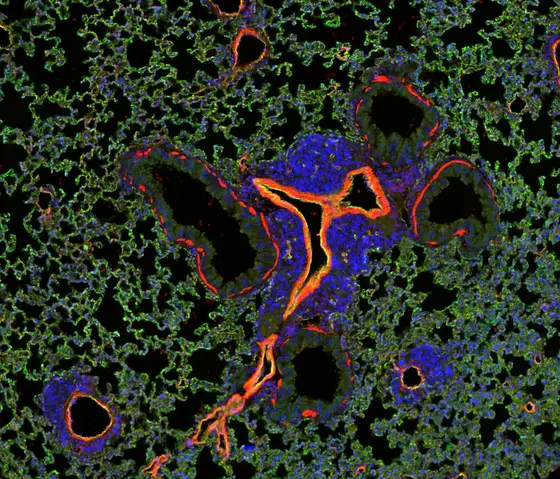
Caption: Metastatic growth in the lungs (mouse): Tumor cells (blue) grow around blood vessels in the lungs (red) to invade into the normal lung tissue (green).
Note on use of images related to press releases
Use is free of charge. The German Cancer Research Center (Deutsches Krebsforschungszentrum, DKFZ) permits one-time use in the context of reporting about the topic covered in the press release. Images have to be cited as follows: “H. Augustin / DKFZ“.
Distribution of images to third parties is not permitted unless prior consent has been obtained from DKFZ's Press Office (phone: ++49-(0)6221 42 2854, E-mail: presse@dkfz.de). Any commercial use is prohibited.