Liver cancer (hepatocellular carcinoma) used to be among the less common cancer types in Germany. In recent decades, however, the numbers of people diagnosed with this disease have also been rising in this country. People who suffer from liver cirrhosis, hepatitis B or C, obesity, or type 2 diabetes mellitus are particularly at risk of developing liver cancer. Liver cancer most frequently develops as a consequence of chronic liver disease, which has become increasingly common in Germany.
An international team of researchers led by Mathias Heikenwälder and his collaboration partner, Achim Weber from Zurich University, have now discovered that an enzyme called caspase 8 plays an important dual role in this process. The studies were performed in mice as a first step. Patient data show that the results can be transferred to humans.
On the one hand, caspase 8 is important for the process of programmed cell death, or apoptosis. Cells that have undergone malignant transformation eliminate themselves by apoptosis in order to protect the organism. Therefore, a motto held for a long time was: Apoptosis protects from cancer. The current study shows that this only holds true for each individual cell and not for whole tissues.
If too many cells at a time undergo apoptosis, this promotes the development of cancer. The reason is that the remaining hepatic cells have to divide at much higher rates in order to make up for lost tissue. “Hepatic cells are not used to high division rates, they cannot cope and make mistakes,“ explained Mathias Heikenwälder from the German Cancer Research Center (Deutsches Krebsforschungszentrum, DKFZ) in Heidelberg.
Patients with chronic inflammation of the liver accumulate high levels of DNA damage, which is fertile ground for cancer. The more mutations have accumulated in a cell's DNA, the more probable it gets that the cell will break out from its normal life cycle and start proliferating and growing out of control.
However, caspase 8 has yet another, completely different function. The molecule is part of a newly identified larger complex that recognizes damage in DNA and triggers repair mechanisms. The functions in apoptosis and repair operate independently of each other. They can also be influenced separately from each other.
This is particularly important for the treatment of liver cancer and chronic liver disease. While complete elimination of the caspase 8 enzyme would prevent programmed cell death and the development of cancer, it would also rob the cell of a DNA repair mechanism. This effect must be avoided.
In a next step, the scientists plan to investigate whether similar processes also proceed in other types of cancer and to study the dynamics of this mechanism in more detail. “So far, we do not know when and why caspase 8 and the other molecules team up to search for DNA damage,“ Heikenwälder said. “Many questions are still unanswered.“
Yannick Boege, Mohsen Malehmir, Marc E. Healy, Kira Bettermann, Anna Lorentzen, Mihael Vucur, Akshay K. Ahuja, Friederike Böhm, Joachim Mertens, Yutaka Shimizu, Lukas Frick, Caroline Remouchamps, Karun Mutreja, Thilo Kähne, Devakumar Sundaravinayagam, Monika Wolf, Hubert Rehrauer, Christiane Koppe, Tobias Speicher, Susagna Padrissa-Altés, Renaud Maire, Jörn M. Schattenberg, Juseong Jeong, Lei Liu, Stefan Zwirner, Regina Boger, Norbert Hüser, Roger J. Davis, Beat Müllhaupt, Holger Moch, Henning Schulze-Bergkamen, Pierre-Alain Clavien, Sabine Werner, Lubor Borsig, Sanjiv A. Luther, Philipp Jost, Ricardo Weinlich, Kristian Unger, Axel Behrens, Laura Hillert, Christopher Dillon,, Michela Di Virgilio, David Wallach, Emmanuel Dejardin, Lars Zender, Michael Naumann, Henning Walczak, Douglas R. Green, Massimo Lopes, Inna Lavrik, Tom Luedde, Mathias Heikenwalder, Achim Weber: A dual role of caspase 8 in triggering and sensing proliferation-associated DNA damage, a key determinant of liver cancer development.
Cancer Cell 2017, DOI: 10.1016/j.ccell.2017.08.010
A picture is available at:
Mcl1_liver.jpg
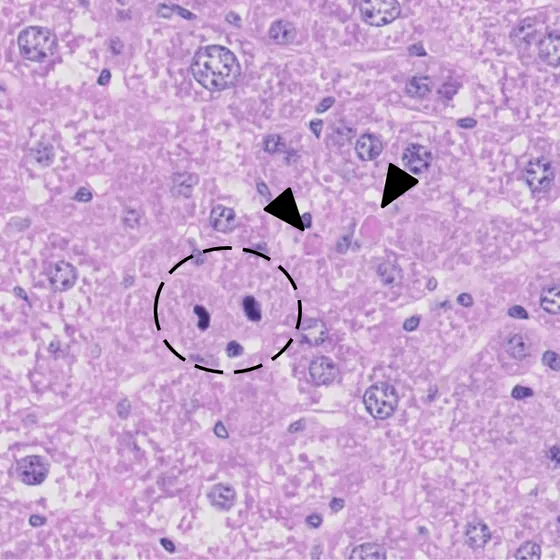
Caption: A model for chronic liver disease: In this genetically modified mouse liver apoptosis is increased. Apoptotic cells are marked by arrow heads, the circle marks mitotic cells.
Note on use of images related to press releases
Use is free of charge. The German Cancer Research Center (Deutsches Krebsforschungszentrum, DKFZ) permits one-time use in the context of reporting about the topic covered in the press release. Images have to be cited as follows: “Source: Heikenwälder/DKFZ“.
Distribution of images to third parties is not permitted unless prior consent has been obtained from DKFZ's Press Office (phone: ++49-(0)6221 42 2854, E-mail: presse@dkfz.de). Any commercial use is prohibited.