Therapy monitoring of novel therapeutic protocols for patient stratifications
- 1. Longitudinal PET-CT studies with FDG in patients with metastatic melanomas under immunotherapy
- 2. Longitudinal PET-CT studies with FDG in patients with unresectable lung tumors under immunotherapy or combined chemo-immunotherapy as well as in resectable lung tumors in a neoadjuvant setting
- 3. A randomized phase III non-inferiority trial assessing lenalidomide,bortezomib and dexamethasone induction therapy with either intravenous or subcutaneous isatuximab in transplant-eligible patients with newly diagnosed multiple myeloma(GMMG HD-8 trial)
- 4. Longitudinal PET-CT studies with FDG in patients with lymphomas under CART cell therapy
1. Longitudinal PET-CT studies with FDG in patients with metastatic melanomas under immunotherapy
In quest of identifying reliable biomarkers for monitoring and prediction of outcome to immunotherapy, we prospectively investigate the potential role of 18F-FDG PET/CT in the management of melanoma patients under treatment with different immune checkpoint inhibitors (ICIs) like CTLA-4, PD1 and LAG-3 inhibitors or their combinations. In order to address these issues, different evaluation methods and acquisition protocols of PET/CT are used and longitudinal studies are performed at different standardized time-points during treatment. Their validation is based on long-term patient clinical and survival data.
The basic research questions, with which this project deals, are the following:
- Impact of 18F-FDG PET/CT early during the course of treatment for prediction of long-term response to immunotherapy in metastatic melanoma
- Novel approaches and dedicated immune-related response criteria for the reliable interpretation of 18F-FDG PET/CT immunotherapy response
- Use of 18F-FDG PET/CT as a reliable marker for the discontinuation of immunotherapy
- Visualization and impact of immune-related adverse events
- Prognostic value of the 18F-FDG uptake in lymphoid organs, like the spleen, as well as their changes during immunotherapy
Cooperation with Prof. Dr. med. Jessica Hassel, Department of Dermatology and National Center for Tumor Diseases (NCT) and University Hospital Heidelberg
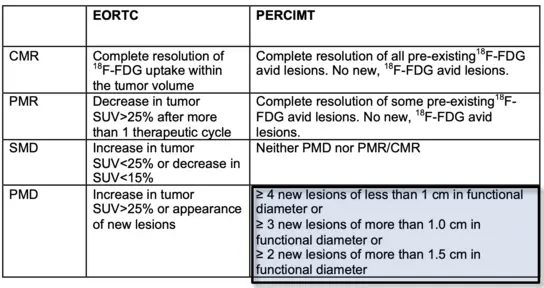
Fig 1a. "Joint EANM/ANZNM practical guidelines/SNMMI procedure standards on recommended use of [18F]FDG PET/CT imaging during immunomodulatory treatments in patients with solid tumors" Ver. 1.0 (Lopci E et al. Eur J Nucl Med Molecular Imaging 2022; 49:2323-2341)
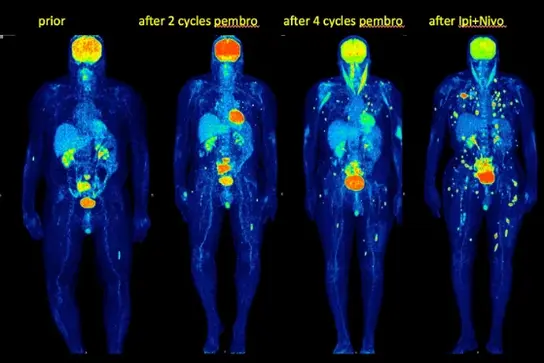
Fig 1b. Definition of new criteria for immunotherapy monitoring with FDG PET -CT PET Response Evaluation Criteria for Immunotherapy (PERCIMT) (Anwar H et al. Eur J Nucl Med Molecular Imaging 2018; 45:376-383)
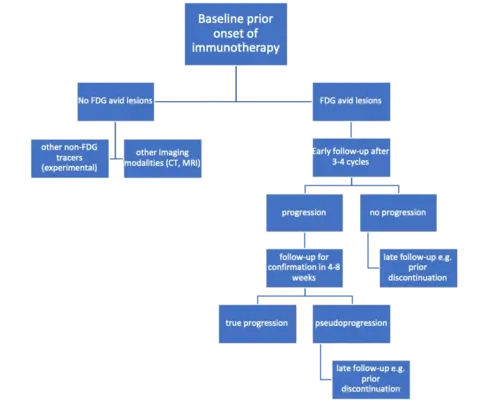
Fig 1c. 18F-FDG PET-CT for immunotherapy monitoring (Dimitrakopoulou-Strauss A et al. Cancer Biother Radiopharm 2023; 38:225-231)
2. Longitudinal PET-CT studies with FDG in patients with unresectable lung tumors under immunotherapy or combined chemo-immunotherapy as well as in resectable lung tumors in a neoadjuvant setting
Patients with metastatic NSCLC or SCLC (group 1) and patients with resectable NSCLC (group 2) will be examined with dynamic PET/CT and 18F-FDG over time. Group 1 will be studied before first-line treatment with a PD-1/PD-L1 inhibitor as monotherapy or in combination with chemotherapy and in a standardized follow-up after the start of therapy. Group 2 will be examined before the start of neoadjuvant combined immunotherapy and chemotherapy and after completion of the preoperative cycles and after completion of all therapy.
The goal of this study is to assess the suitability of dynamic PET/CT as early quantitative imaging modality to detect progression/response by taking the clinical data into account. Reference will be the progression-free survival (PFS)and overall survival (OS) for both groups. In group 2, the pathological remission will also be used to evaluate the response to neoadjuvant therapy (pCR [i.e. 0% viable tumor tissue], MPR [i.e. <10% viable tumor tissue]).
Cooperation with Prof. Dr. med. Michael Thomas, head of the Internal Medicine - Oncology Department at the Heidelberg Thorax Clinic.
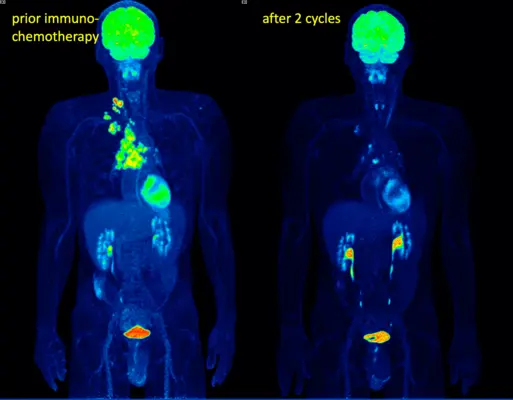
Fig 2. Early response after 2 cycles of combined immuno- and chemotherapy in primary non-resectable lungcancer
3. A randomized phase III non-inferiority trial assessing lenalidomide,bortezomib and dexamethasone induction therapy with either intravenous or subcutaneous isatuximab in transplant-eligible patients with newly diagnosed multiple myeloma(GMMG HD-8 trial)
Multiple myeloma often shows heterogeneous involvement in the bone marrow and in extramedullary foci in imaging procedures. Imaging methods such as functional magnetic resonance imaging (MRI) and positron emission tomography - computed tomography (PET/CT) allow complementary non-invasive diagnostics and provide information on the damage to the mineralized bone, the morphology and the pathophysiology of the myeloma cells and their surroundings.
The objectives of this study are:
- Acquisition of high-quality, comparable quantitative data from diffusion-weighted MRI and PET/CT whole-body examinations at baseline and during the course of therapy
- Detection of resistant lesions during study therapy
- Determination of the prognostic significance of MRI and PET/CT in PFS and OS, particularly in comparison to parallel analyzes of minimal residual disease (MRD) from iliac crest aspirates.
Cooperation with Prof. Dr. med. Hartmut Goldschmidt, Department of Internal Medicine V, University Hospital Heidelberg, Heidelberg, Germany; National Center for Tumor Diseases Heidelberg, Heidelberg
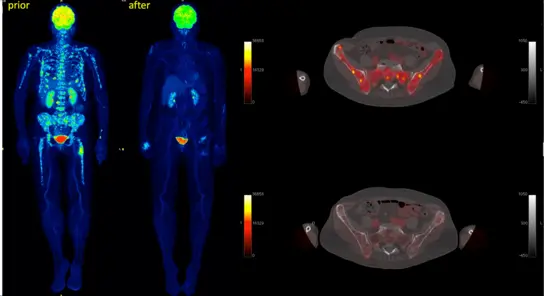
Fig 3. Early response after induction chemotherapy in multiple myeloma (Sachpekidis C et al. Haematologica. 2019; 104:e420-e423)
4. Longitudinal PET-CT studies with FDG in patients with lymphomas under CART cell therapy
Car-T cell therapy as a new cellular immunotherapy offers great opportunities for patients while at the same time presenting new challenges for imaging. FDG-PET/CT represents the gold standard for imaging aggressive lymphomas such as large B-cell lymphoma (DLBCL) and has therefore also been used as the standard in studies of Car-T cell therapy. The metabolic assessment of glucose uptake using PET/CT is significantly superior to the morphological assessment using CT and is therefore recommended across specialist societies for staging and follow-up of large B-cell lymphoma. Diagnostic advantages of MRI are better assessment of bone or bone marrow infiltration and independence from glucose metabolism. Finally, the lack of radiation exposure enables closer therapy monitoring.
The aims of the study are:
- to evaluate and compare PET/CT and whole-body MRI in patients receiving Car-T cell for therapy assessment
Cooperation with Prof. Dr. med. Peter Dreger, Department of Internal Medicine V, University Hospital Heidelberg, Heidelberg, Germany; National Center for Tumor Diseases Heidelberg, Heidelberg and the Radiology Department of DKFZ
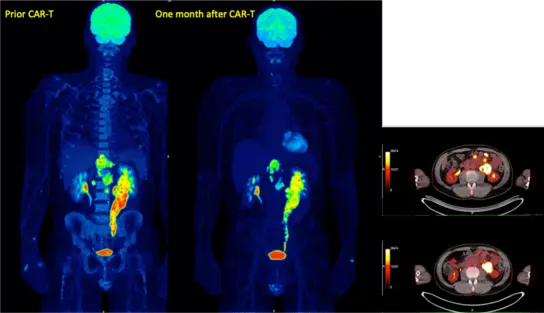
Fig 4. Early response one month after CAR-T cell therapy in resistant diffuse large B-cell lymphoma