Molds of the genus Apergillus produce dreaded mycotoxins, but also substances with interesting pharmacological effects. One of these substances is fumagillin, which is produced by the common fungus Aspergillus fumigatus. Over 20 years ago, American researchers discovered by chance that fumagillin inhibits the growth of cancer cells, mainly by blocking the development of the tumor’s blood vessels. Since the animals that had been treated with the substance also lost a great deal of weight, the researchers realized that fumagillin may also be used to treat obesity.
However, in further pre-clinical and clinical experiments the promising compound revealed substantial inadequacies. It turned out to be very unstable and caused serious side effects in the central nervous system.
“Many important drugs, particularly antibiotics and anticancer drugs, are based on natural compounds," says Aubry Miller, who leads the research group Cancer Drug Development at the German Cancer Research Center (Deutsches Krebsforschungszentrum, DKFZ) together with his colleague Nikolas Gunkel. “However, the original substances often need to be chemically optimized to improve their effectiveness and their pharmacological properties."
To this end, chemists usually modify the natural compound. This is how they made, for example, the agent beloranib – a modified version of fumagillin that causes fewer side effects than the original natural compound but is similarly unstable.
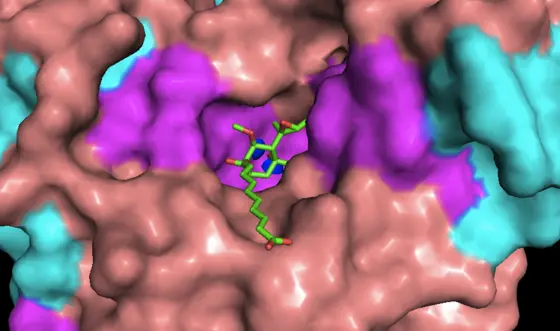
The DKFZ researchers, collaborating with colleagues from the Institute of Pharmacy and Molecular Biotechnology at the University of Heidelberg, therefore chose a different approach. They synthesized a molecule that mimics fumagillin’s three-dimensional structure without being chemically related to the fungal substance. “Fumagillin acts by blocking a specific enzyme in our cells," says Michael Morgen, who is a first author of the publication. “Our goal was to synthesize a compound which has a shape that is similar to fumagillin, thereby enabling it to block this same enzyme. As a consequence, this compound should have biological properties that are also similar to fumagillin. It was very exciting to see, using X-ray crystallography, that our compound binds to the exact same amino acid in the active site of the enzyme that fumagillin binds to."
The synthetic compound does inhibit the growth of blood vessels cells as well as tumor cells, but it is slightly less effective than the natural compound. However, the stability of the substance proved to be significantly superior to the original natural compound.
“Many biological tests are still ahead of us," Miller says. “For example, we do not know anything yet about the side effects in the central nervous system. We regard our molecule as a starting compound that can be further optimized." In view of its potential range of uses – against different cancers as well as against obesity – the researchers are optimistic of their compound’s potential.
Michael Morgen, Christian Jöst, Mona Malz, Robert Janowski, Dierk Niessing, Christian D. Klein, Nikolas Gunkel, Aubry K. Miller: Spiroepoxytriazoles Are Fumagillin-like Irreversible Inhibitors of MetAP2 with Potent Cellular Activity. ACS Chemical Biology 2016, DOI: 10.1021/acschembio.5b00755