Proteins are made of long chains of amino acids. In order to perform their often vital functions, each chain of amino acids must fold into a particular three-dimensional structure. If growth conditions change, such as by a rise in environmental temperature, proteins may lose their structure and unfold. Then there is a danger of the unfolded protein chains clumping together. “Such aggregated proteins can no longer perform their tasks", says Prof. Dr. Bernd Bukau, who heads a joint research department of the Center for Molecular Biology (ZMBH) at Heidelberg University and the German Cancer Research Center (DKFZ). “This loss of function can lead to cell death as it does, for example, in neurodegenerative diseases such as Alzheimer’s and Parkinson’s or in aging processes."
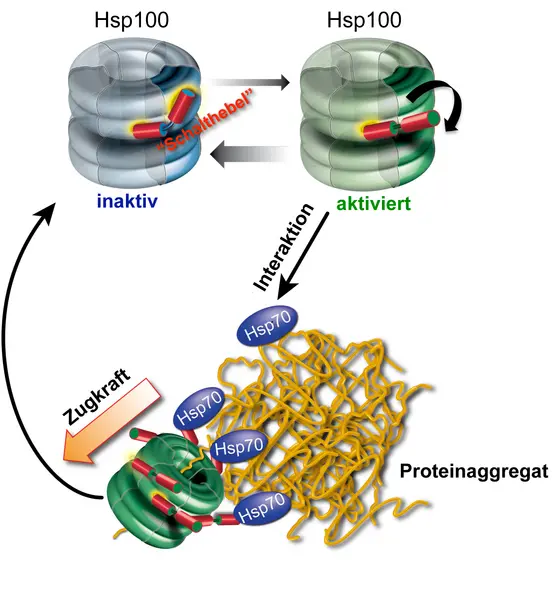
However, aggregation does not necessarily have to be the end of a protein’s life cycle. “Cells have repair systems called molecular chaperones, which can even dissolve and refold aggregated proteins," says Associate Professor (PD) Dr. Axel Mogk, who is also a member of both ZMBH and DKFZ. A team of two chaperones known as Hsp70 and Hsp100 carry out the “repair". The Heidelberg scientists were able to show that Hsp70 regulates the activity of the Hsp100 chaperone using an inbuilt molecular switch.
This switch is initially in “off" position, meaning that it curbs energy consumption and hence the activity of Hsp100. As soon as team partner Hsp70 spots an aggregated protein, it throws the switch into “on" position, thereby activating Hsp100 on the spot. In this state, the “motor" of the ring-shaped Hsp100 protein starts and can pull out individual chains from the protein aggregate. The disaggregated, unfolded protein then has a chance to reassume its original structure and become functional again. The Heidelberg scientists could also show that the inbuilt switch plays an essential role in this complicated repair system, because its loss in permanently active Hsp100 variants leads to cell death.
The research work is a project of the DKFZ-ZMBH Alliance, which is a strategic collaboration of the German Cancer Research Center (Deutsches Krebsforschungszentrum, DKFZ) and the Center for Molecular Biology (ZMBH) at Heidelberg University. The Heidelberg Institute for Theoretical Studies (HITS) focuses on new theoretical approaches towards interpreting the rapidly increasing amount of experimental data.
Original publications:
F. Seyffer, E. Kummer, Y. Oguchi, J. Winkler, M. Kumar, R. Zahn, V. Sourjik, B. Bukau & A. Mogk: Hsp70 proteins bind Hsp100 regulatory M domains to activate AAA+ disaggregase at aggregate surfaces, Nature Structural & Molecular Biology, 18 November 2012, doi: 10.1038/nsmb.2442
Y. Oguchi, E. Kummer, F. Seyffer, M. Berynskyy, B. Anstett, R. Zahn, R.C. Wade, A. Mogk & B. Bukau: A tightly regulated molecular toggle controls AAA+ disaggregase, Nature Structural & Molecular Biology, 18 November 2012, doi: 10.1038/nsmb.2441