CCU Molecular Hematology/Oncology - Clinical Research
Cancer of unknown primary (CUP) accounts for 2–5% of all malignancies, with 80–85% being of an unfavourable subset with extensive metastatic dissemination. There has been little progress in improving outcomes for patients with CUP. The unfavourable subset has a poor prognosis, with a median overall survival of less than 1 year when treated with the current standard of care (non-specific platinum-based chemotherapy), creating a high unmet medical need for new therapeutic approaches.

To advocate for the interests of patients and families impacted by CUP, we partner with patient advocates on a national and international level and encourage everyone interested to get in touch using the following links:

World CUP Alliance:
An international Alliance of all those patient advocates that are focused on improving diagnostics and treatments for all patients affected by CUP.

CUP Forum:
A German patient advocacy organization offering support and useful information for people affected by CUP.

CUPISCO trial
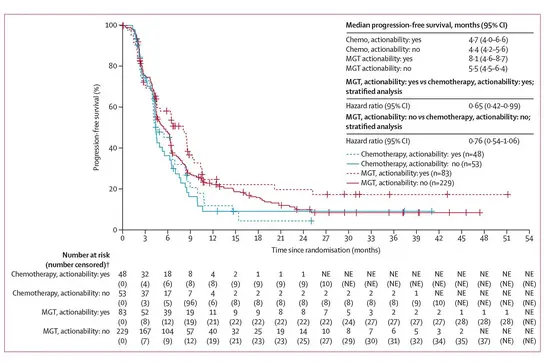
To determine the efficacy and safety of molecularly targeted treatments or immunotherapy guided by genomic profiling, we conducted an international, randomized, multi-center phase II study (CUPISCO, NCT03498521; Krämer et al, Lancet, 2024). The trial was performed at 159 cancer centers in 34 countries and included 636 patients with newly diagnosed CUP syndrome, constituting the largest clinical trial ever conducted in patients with CUP. Following comprehensive genomic testing and randomization, trial participants gained access to targeted therapies and immunotherapy based on their unique tumor profiles.
Molecularly guided treatments significantly improved survival, proving the efficacy of targeted therapies and immunotherapy in a tumor-agnostic setting. CUPISCO was the first phase II trial to implement targeted treatments irrespective of the tumor tissue of origin, constituting a major milestone in precision oncology. Alongside with the recommendation for comprehensive molecular profiling in all patients with CUP, the final results of the CUPISCO trial led to refined diagnostic algorithms that were included into the ESMO Guidelines for the Diagnosis and Treatment of CUP.
Kaplan-Meier plot showing the progression-free survival of CUP patients treated with targeted and immunotherapy versus standard chemotherapy in the CUPISCO trial.
CheCUP trial
Our revious work has indicated the efficacy of immunotherapy in CUP: in a national, multicenter phase II trial (CheCUP trial, EudraCT 2018-004562-33; Pouyiourou, Kraft et al, Nat Commun, 2023), patients suffering from CUP syndrome relapsed or refractory after platinum-based chemotherapy were treated with immune checkpoint inhibitors using combined Ipilimumab and Nivolumab.
High tumor mutational burden (TMB) emerged as a predictive biomarker of superior treatment response and survival, offering the option of immunotherapy to patients with tumors harboring this feature.
Kaplan-Meier plot showing the progression-free (left) and overall survival (right) of CUP patients with high versus low tumor mutational burden treated with immunotherapy in the CheCUP trial.

SACICUP trial
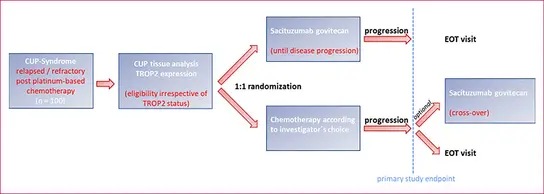
The SACICUP trial, a national, multicenter, randomized phase II trial, will determine the efficacy and safety of sacituzumab-govitecan – an antibody drug conjugate directed against the cell surface protein TROP2 – in patients suffering from CUP syndrome relapsed or refractory after platinum-based chemotherapy. The study is scheduled to start in early 2025 in several German centers.
SACICUP study design.

Our CUP Clinical Trials Office
Since we have been running a special consultation for patients with CUP syndrome together with the National Center for Tumor Diseases (NCT) for 15 years, we can quickly provide CUP patients with new diagnostic and therapeutic approaches.

Medical Doctors

Prof. Dr. Alwin Krämer
Principal Investigator (PI)
Phone 1: +49 6221 / 56-38183
Phone 2: +49 6221 / 42-1440


Publications (selected)
Pouyiourou M, Bochtler T, Pauli C, Moch H, Brobeil A, Pantel K, Stenzinger A, Krämer A
Molecularly guided therapy versus chemotherapy after disease control in unfavourable cancer of unknown primary (CUPISCO): an open-label, randomised, phase 2 study.
Krämer A, Bochtler T, Pauli C, Shiu KK, Cook N, Janoski de Menezes J, Pazo-Cid RA, Losa F, Robbrecht DGJ, Tomášek J, Arslan C, Özgüroğlu M, Stahl M, Bigot F, Kim SY, Naito Y, Italiano A, Chalabi N, Durán-Pacheco G, Michaud C, Scarato J, Thomas M, Ross JS, Moch H, Mileshkin L
Tumour mutational burden: clinical utility, challenges and emerging improvements.
Budczies J, Kazdal D, Menzel M, Beck S, Kluck K, Altbürger C, Schwab C, Allgäuer M, Ahadova A, Kloor M, Schirmacher P, Peters S, Krämer A, Christopoulos P, Stenzinger A
Recommendations for the use of next-generation sequencing (NGS) for patients with advanced cancer in 2024: a report from the ESMO Precision Medicine Working Group.
Mosele MF, Westphalen CB, Stenzinger A, Barlesi F, Bayle A, Bièche I, Bonastre J, Castro E, Dienstmann R, Krämer A, Czarnecka AM, Meric-Bernstam F, Michiels S, Miller R, Normanno N, Reis-Filho J, Remon J, Robson M, Rouleau E, Scarpa A, Serrano C, Mateo J, André F
Cancer of unknown primary derived from regressed breast cancer.
Pouyiourou M, Mokry T, Feszler M, Teifke A, Kreft A, Krämer A
Baseline mutational profiles of patients with carcinoma of unknown primary origin enrolled in the CUPISCO study.
Westphalen CB, Federer-Gsponer J, Pauli C, Karapetyan AR, Chalabi N, Durán-Pacheco G, Beringer A, Bochtler T, Cook N, Höglander E, Jin DX, Losa F, Mileshkin L, Moch H, Ross JS, Sokol ES, Tothill RW, Krämer A
Nivolumab and ipilimumab in recurrent or refractory cancer of unknown primary: a phase II trial.
Pouyiourou M*, Kraft BN*, Wohlfromm T, Stahl M, Kubuschok B, Löffler H, Hacker UT, Hübner G, Weiss L, Bitzer M, Ernst T, Schütt P, Hielscher T, Delorme S, Kirchner M, Kazdal D, Ball M, Kluck K, Stenzinger A, Bochtler T, Krämer A
Modified study designs to expand treatment options in personalised oncology: a multistakeholder view.
Le Tourneau C, Andre F, Helland A, Mileshkin L, Minnaard W, Schiel A, Tasken K, Thomas DM, Veronese ML, Duran-Pacheco G, Leyens L, Rufibach K, Thomas M, Krämer A
Cancer of unknown primary: ESMO Clinical Practice Guideline for diagnosis, treatment and follow-up.
Krämer A, Bochtler T, Pauli C, Baciarello G, Delorme S, Hemminki K, Mileshkin L, Moch H, Oien K, Olivier T, Patrikidou A, Wasan H, Zarkavelis G, Pentheroudakis G, Fizazi K, on behalf of the ESMO Guidelines Committee
Cancer-of-unknown-primary-origin: A SEER – Medicare study of patterns of care and outcomes among elderly patients in clinical practice.
Mileshkin L, Bochtler T, Gatta G, Kurzrock R, Beringer A, Müller-Ohldach M, Surinach A, Perret C, Thomas M, Gondos A, Krämer A
Local ablative treatment with surgery and/or radiotherapy in single-site and oligometastatic carcinoma of unknown primary (CUP).
Pouyiourou M, Wohlfromm T, Kraft B, Hielscher T, Stichel D, von Deimling A, Delorme S, Endris V, Neumann O, Stenzinger A, Krämer A, Bochtler T
A challenging task – Identifying patients with cancer of unknown primary (CUP) according to ESMO guidelines: the CUPISCO trial experience.
Pauli C, Bochtler T, Mileshkin L, Baciarello G, Losa F, Ross J, Pentheroudakis G, Zarkavelis G, Yalcin S, Özgüroğlu M, Beringer A, Scarato J, Mueller-Ohldach M, Thomas M, Moch H, Krämer A
Comprehensive genomic profiling of carcinoma-of-unknown-primary-origin: retrospective molecular classification considering the CUPISCO study design.
Ross JS, Sokol ES, Moch H, Mileshkin L, Baciarello G, Losa F, Beringer A, Thomas M, Elvin J, Ngo N, Jin DX, Krämer A
Adding cetuximab to paclitaxel and carboplatin for first-line treatment of carcinoma of unknown primary (CUP): results of the phase II PACET-CUP trial.
Folprecht G, Trautmann K, Stein A, Hübner G, Stahl M, Kasper S, Kretzschmar A, Köhne CH, Grünwald V, Hofheinz R, Schütte K, Löffler H, Bokemeyer C, Krämer A, Arbeitsgemeinschaft Internistische Onkologie (AIO) - CUP group