Immune Modulation in Cancer

Dr. Martina Seiffert
Our research group investigates tumor immunology and the microenvironment of non-Hodgkin B-cell lymphomas as well as brain metastases from breast and lung cancer, focusing on cancer-induced immunosuppression and T-cell exhaustion to develop novel immunotherapies in patient-based models.

Our research
My group is interested in tumor immunology and the microenvironment in non-Hodgkin's B-cell lymphoma and brain metastases from breast and lung cancer. In particular, we investigate mechanisms of cancer-induced immunosuppression and T-cell exhaustion as pathological factors in cancer. Our aim is to identify mediators and molecular mechanisms of interactions between cancer cells and their environment, focusing on immune cells of the myeloid and lymphoid lineages. We use our findings to develop and test novel immunotherapy approaches in patient-based coculture models and genetic mouse lines.
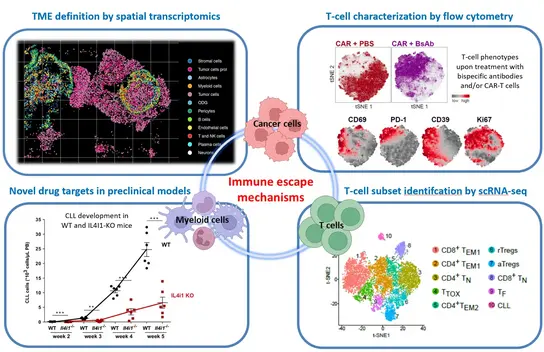
The complex cellular and molecular composition of tumors is essential for their pathobiology. We have previously identified molecular mechanisms and candidate molecules responsible for cancer-induced remodeling of the microenvironment and immune system.
Our current work focuses on an in-depth characterization of immune and stromal cells in B-cell lymphomas and brain metastases, with the aim of elucidating novel pro-tumorigenic and immunosuppressive mechanisms, as well as identifying candidate genes and signaling pathways for the development of new therapeutic approaches. This also includes the definition of new prognostic or predictive biomarkers and the identification of mechanisms of therapy resistance. We are particularly interested in cancer-associated T cell exhaustion, the immunosuppressive function of myeloid cells, and the mediators responsible for these two phenotypes. Ongoing projects focus on interleukin-10 (IL-10), interleukin-4-induced-1 (IL4I1) and galectin-9.
We use single cell transcriptome analysis, spectral flow cytometry and proteomic analysis to study primary tumor tissue and blood cells, multiplex immunofluorescence staining and in situ transcript quantification of tissue sections to spatially map the tumor microenvironment, as well as functional assays and treatment studies in vitro and in mouse models to validate the relevance of identified candidate genes and pathways. Our preclinical therapy studies also include the optimization of the treatment of CLL and DLBCL with CAR T-cell products and bispecific antibodies.
To this end, we cooperate with academic and industry partners and are in close contact with our colleagues in the clinic.
Team

Selected publications
Longitudinal omics data and preclinical treatment suggest the proteasome inhibitor carfilzomib as therapy for ibrutinib-resistant CLL.
Arseni L, Sigismondo G, Yazdanparast H, Hermansen JU, Mack N, Ohl S, Kalter V, Iskar M, …, Zapatka M, Roessner PM, Tausch E, Stilgenbauer S, Dietrich S, Savitski MM, Skånland SS, Krijgsveld J, Lichter P, Seiffert M.
Roessner PM, Seufert I, …., Rippe K, Seiffert M.
Brinkmann BJ, Floerchinger A, Schniederjohann C, Roider T, Coelho M, Mack N, Bruch P-M, Liebers N, Dötsch S, Busch DH, Schmitt M, Neumann F, Roessner PM, Seiffert M, Dietrich S.
Hanna BS, Llao Cid L, Iskar M, Roessner PM, Klett L, Wong JKL, …, Rippe K, Feuerer M, Ramsay A, Lichter P, Zapatka M, Seiffert M.
Sadik A, Somarribas Patterson LF, Öztürk S, Mohapatra SR, Panitz V, Secker PF, …, Böhme A, Schäuble S, Thedieck K, Trump S, Seiffert M, Opitz CA.
Reviews
Floerchinger A, Seiffert M.
Roessner PM, Seiffert M.
Hanna BS, Öztürk S, Seiffert M.
Get in touch with us
