GMP & T Cell Therapy
- Immunology, Infection and Cancer

Prof. Dr. Stefan Eichmüller
Arbeitsgruppenleiter/ group leader
Immunotherapeutics represent promising treatment options against cancer as shown by the therapeutic success of checkpoint inhibitors. The “GMP & T cell therapy” research group explores T cell mediated tumor rejection and develops new concepts for clinical applications.

Our Research
Our research group investigates various possibilities for optimizing T cell-mediated tumor defense and prepares their application together with clinical cooperation partners. In our translational research, we investigate various aspects of the T cell-mediated immune response.
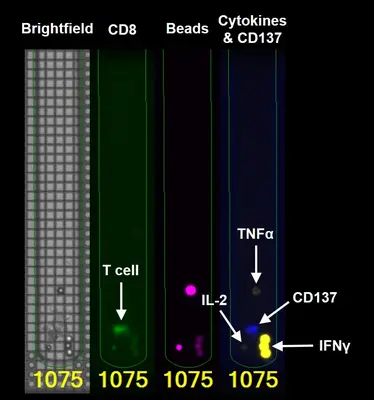
One important aspect is the detailed analysis of individual T cells from the tumor with regard to their ability to recognize and destroy tumor cells. The Bruker Lightning System enables such single cell analyses on a chip. Here, 1500 T cells in individual chambers of the chip are examined in parallel for their reactivity against autologous tumor cells (i.e. tumor cells from the same patient). Reactive T cells can then be exported individually from the chip to determine the genetic sequence of the reactive T cell receptor. The identified TCR gene is cloned and expressed in autologous T cells. The resulting TCR-T cells are tested for the functional recognition of autologous tumor cells. This approach is currently being used in hematological tumors (multiple myeloma) and in solid tumors such as ovarian cancer (see project “Tumor-specific TCRs”).
Solid tumours in particular are often characterized by an immunosuppressive tumour environment, which is maintained by the accumulation of suppressive cell types and inhibitory factors released by the tumour. Another project of our research group is therefore investigating miRNAs that can influence the immunosuppressive tumor environment (see project “miRNAs”).
The analysis of specific T cell responses requires synthetic peptides, which are produced in our peptide laboratory (see “Peptide Unit”). In parallel, the team of the Peptide Unit is currently establishing GMP-compliant peptide production and quality control for use in patients as part of vaccination studies.
Our GMP team is currently optimizing production lines for the manufacture of cellular products for adoptive T cell transfer. The production lines include the expansion of tumor-infiltrating lymphocytes, the production of CAR T cells and TCR transgenic T cells (see “GMP Unit”). This is done in close collaboration with Prof. Dirk Jäger's team at the NCT. A new GMP facility with cleanrooms for cell production for clinical trials is currently under construction.
Projects
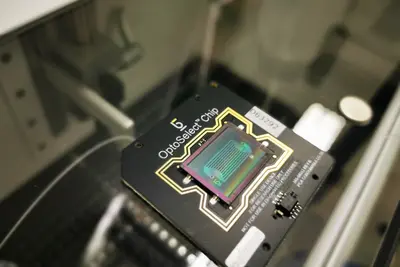
Adoptive T cell transfer is currently considered the most successful treatment strategy in cancer therapy, provided that the transferred T cells recognize mutated epitopes, so-called neo-epitopes, presented by tumor cells. The major challenges of adoptive T cell therapy consitst in the definition of tumor-specific targets and the direct identification of tumor-specific T cells and their T cell receptor (TCR). In this collaborative project, we aim to directly identify myeloma-specific T cells isolated from the bone marrow of patients. Since the bone marrow aspirates contain both tumor cells and T cells, they can be used to screen for autologous, tumor-specific T cells. We use the Berkeley Lights “Lightning™ Optofluidic System”, a benchtop device that allows individual analysis of several thousand cells in separate nanopins on a chip. The functional interaction between sorted myeloma cells and individual T cells is analyzed by quantifying the cytokines released by reactive T cells. In a next step, the T cells identified in this way are isolated from the chip for TCR sequencing and DNA vectors are generated that code for the identified TCRs. Finally, the tumor reactivity of these TCRs against the autologous myeloma cells is tested. Ultimately, such strategies could be used to generate autologous TCR-transgenic T cells for the adoptive T cell therapy of patients with multiple myeloma. Meanwhile we have extended the application of the Berkeley Lights “Lightning™ Optofluidic System” to solid tumors such as ovarian and colorectal cancer.
Another aspect of our research activities deals with the identification and characterization of pan-functional miRNA molecules (miRNAs).
MicroRNAs (miRNAs) are small, non-coding RNA molecules that specifically bind to mRNA sequences of certain genes and thereby inhibit their expression at the post-transcriptional level. Tumor cells often exhibit a deregulated miRNA expression pattern in which both tumor growth-promoting (oncomiRs) and tumor-suppressive miRNAs (tumor suppressor miRs) play a role. In tumor-associated macrophages (TAMs), miRNAs have also been described that are overexpressed in immune-stimulatory (M1-like) or immunosuppressive (M2-like) TAMs, respectively, and also drive functional polarization into M1- or M2-like TAMs.
Based on miRNA library screening and molecular biological investigations (flow cytometry, qPCR, Western blot, reporter assays) in human tumor cell lines and primary macrophages, we identify and characterize specific miRNAs that induce anti-tumor effects in both tumor cells and tumor-associated macrophages (TAMs) and are therefore referred to as pan-functional miRNAs. Pan-functional miRNAs may therefore have a therapeutic enhancer effect that could be of clinical significance.

The GMP Unit enables the production of in vitro expanded immune cells for clinical studies. The prerequisite for use as an investigational medicinal product is a manufacturing authorization in accordance with the German Medicinal Products Act (AMG), which permits the isolation, purification and expansion of immune cells from the patient. In the past, the GMP unit has received a manufacturing authorization for “autologous ex vivo reactivated tumor-specific T cells for adoptive T cell transfusion” and for “autologous ex vivo expanded tumor-infiltrating cells for adoptive T cell transfusion”. The GMP unit is currently working on the optimization of expansion protocols for transgenic and non-transgenic autologous T cells. In collaboration with Prof. Dirk Jäger, a new Center for Innovative Therapies (CIT) is founded, which will provide cleanrooms for the GMP-compliant synthesis and filling of complex peptide vaccines, cleanrooms for the production of T cell preparations and laboratories for the molecular characterization and quality control of investigational medicinal products.
Production and quality control of synthetic peptides for research
The development of novel immunotherapies is often dependent on the rapid and cost-effective production of short (8 - 11 aa for MHC I epitopes) and long (13 - 25 aa for MHC II epitopes) peptides. For this purpose, the GMP and T-cell therapy research group runs its own peptide laboratory (Peptide Unit) with efficient manufacturing processes for the parallel synthesis and purification of several hundred peptides per year.
The synthetized peptides are used in research projects of various DKFZ groups for different purposes, such as the validation of immune epitopes, MHC multimer staining, the production of peptide-loaded antigen-presenting cells (APCs) for functional T cell assays and therapeutic vaccinations of mice.
Peptides are routinely produced from 20 proteinogenic amino acids. However, customer-specific modifications (e.g. fluorophore-coupled peptides, D-amino acids, cyclizations, special building blocks) can also be offered upon request. The peptide laboratory also provides service, e.g. for evaluating producibility, stability and solubility of peptides.
Synthesis and purification
Peptides are produced by solid phase synthesis using the Fmoc strategy on a fully automated multiple synthesizer. The synthesis is carried out on different polymeric carriers (polystyrene, PEG) using suitable activators (carbodiimides, phosphonium and aminium salts). To avoid deletion sequences, incomplete sequences are acetylated after each cycle (capping). After separation of peptides from the carrier with trifluoroacetic acid, raw peptides are optionally purified by preparative reversed-phase chromatography using a water/acetonitrile gradient. Finally, peptides are freeze-dried for optimal stability.
Quality control
All peptides are routinely checked using analytical LC-MS (Dionex UltiMate 3000): purity is determined by chromatographic separation and absorbance at 215 nm, identity is verified on a coupled linear ion trap mass spectrometer (ThermoFisher LTQ XL).
GMP peptides for peptide vaccination
A promising approach for tumor treatment is the use of peptide vaccination cocktails with patient-specific neoepitopes, which can often induce long-lasting immune responses in patients. For human application, however, peptides must be produced under strict standards of Good Manufacturing Practice (GMP), including the use of a clean room environment and extended quality control measures. The peptide laboratory is working on an adapted production process under GMP requirements in order to produce peptides as active substances for clinical studies.
Journal Club Cancer Immunotherapy
We offer a weekly Journal Club on “Cancer Immunotherapy”, which takes place on Mondays at 9.00 am with people from different departments and research programs of the DKFZ and NCT. The seminar participants are Master and PhD students, postdocs and group leaders. Our aim is to constructively discuss the latest literature in the field of cancer immunotherapy. Each participant presents a paper about twice a year in a 30 to 40-minute PowerPoint presentation. The publication is then discussed by all participants. The Journal Club takes place virtually as a video conference followed by a discussion between all participants.
If you are interested in participating, please contact Stefan Eichmüller, who is organizing this seminar. Participation can be confirmed as part of the PhD program.
Teaching
Our team is continuously involved in various teaching activities.
Journal Club Cancer Immunotherapy
We offer a weekly Journal Club on “Cancer Immunotherapy” with participants from different departments and research programs of the DKFZ and NCT as well as the University of Heidelberg. Further details can be found in the Journal Club Cancer Immunotherapy section.
Major Cancer Biology
Together with other departments we organize and head the module HP-F11 “Immunological Methods” which is part of the “Focus Bioscience 1&2”. This includes lecture series and tutorials on “Tumor Immunology, Virology and Cancer” as well as the supervision of students during an internship (Prof. S. Eichmüller, Dr. W. Osen). Further details on the Major Cancer Biology can be found here.
Internship/ Master thesis
Students of the “Molecular Bioscience” Master program at the University of Heidelberg, Faculty of Biosciences, can complete an internship in our group. To ensure optimal supervision, this is limited to one student at a time. It is also possible to write a Master thesis in our group.
Prof. Dr. Stefan Eichmüller
You can find Prof. Eichmüller's CV below as PDF download.

Team GMP & T cell therapy
Our working group consists of different sub-units: a scientific working group and a GMP unit, which are currently located in the main building, as well as a peptide unit in building TP4 of the Technology Park.
For more information please follow the link “Entire team” below.
14 Employees
-

Prof. Dr. Stefan Eichmüller
Arbeitsgruppenleiter/ group leader
-

Dr. Yannic Altrichter
Projektmanager Peptidherstellung/ project manager peptide production
-

Felix Andres
PhD Student/ PhD student
-

Kai Beißer
Technische Assistenz Peptide/ technician peptides
-

Chantal Crocoll
Bachelor Peptide/ B.Sc. peptides
-

Elke Dickes
Technische Assistenz Wissenschaft, GMP/ technician research, GMP
-

Dr. Bettina Meißburger
Projektmanager GMP, Zelltherapie/ project manager GMP, cell therapy
-

Robin Mölle
Technische Assistenz Peptide/ technician peptides
-

Wolfgang Müller
Technische Assistenz GMP/ technician GMP
-

Dr. Wolfram Osen
Gruppenleiter präklinische Forschung/ group leader preclinical research
-

Rainer Schell
Technische Assistenz GMP/ technician GMP
-
Dr. Patrick Schmidt
Gruppenleiter transgene T-Zelltherapie/ group leader transgenic T cell therapy
-

Claudia Sterzik-Luksch
Technische Assistenz GMP/ technician GMP
-

Julian Thimm
Projektmanager GMP Compliance/ project manager GMP compliance
Alumni
Alumni of the research group can be found here.
Publications
2025
- Laurent PA, Andre F, Bobard A, Deandreis D, Demaria S, Depil S, Eichmüller SB, Fernandez-Palomo C, Foijer F, Galluzzi L, Galon J, Guckenberger M, Harrington KJ, Herrera FG, Huber PE, Italiano A, Karam SD, Kroemer G, Lambin P, Leuschner C, Mantovani A, Meylan E, Mondini M, Pittet MJ, Pouget JP, Remon J, Sorensen CS, Sotiriou C, Vanpouille-Box C, Weichselbaum RR, Welsh JW, Zitvogel L, Formenti SC, and Deutsch E (2025) Pushing the boundaries of radiotherapy-immunotherapy combinations: highlights from the 7(th) immunorad conference. Oncoimmunology 14: 2432726.
2024
- Wang Y, Lazaridou M-F, Kordaß T, Massa C, Vaxevanis CK, Riemann D, Eichmüller S, and Barbara S (2024) Unconventional microRNA role: Enhancing the human leukocyte antigen class I antigen processing pathway via interacting with a silencer. Clin Transl Med 14: e70010.
- Schmidt P, Eichmüller SB, Sun Y-F, and Scolnick J (2024) Personalized Immunotherapy: Advancing processes to extend patient collectives. Frontiers Media SA
- Meyer M, Parpoulas C, Barthelemy T, Becker JP, Charoentong P, Lyu Y, Borsig S, Bulbuc N, Tessmer C, Weinacht L, Ibberson D, Schmidt P, Pipkorn R, Eichmüller SB, Steinberger P, Lindner K, Poschke I, Platten M, Fröhling S, Riemer AB, Hassel JC, Roberti MP, Jäger D, Zörnig I, and Momburg F (2024) MediMer: a versatile do-it-yourself peptide-receptive MHC class I multimer platform for tumor neoantigen-specific T cell detection. Front Immunol 14: 1294565. Link to full text
2023
- Kordaß T, Chao T-Y, Osen W, and Eichmüller SB (2023) Novel microRNAs modulating ecto-5′-nucleotidase expression. Front. Immunol. 14. Link to full text
- Pane AA, Kordaß T, Hotz-Wagenblatt A, Dickes E, Kopp-Schneider A, Will R, Seliger B, Osen W, and Eichmüller SB (2023) miRNAs affecting the susceptibility of melanoma cells to CD8+ T cell-mediated cytolysis. Clin Transl Med 13(2): e1186. Link to full text
- Chao T-Y*, Kordaß T*, Osen W, and Eichmüller SB (2022) SOX9 is a target of miR-134-3p and miR-224-3p in breast cancer cell lines. Mol Cell Biochem 478(2):305-315. Link to full text
2022
- Hartmann L, Osen W, Eichmüller OL, Kordaß T, Furkel J, Dickes E, Reid C, Debus J, Brons S, Abdollahi A, Moustafa M, Rieken S, and Eichmüller SB (2022) Carbon ion irradiation plus CTLA4 blockade elicits therapeutic immune responses in a murine tumor model. Cancer Lett. 500. Link to full text
- Kehl N, Kilian M, Michel J, Wagner TR, Uhrig S, Brobeil A, Sester LS, Blobner S, Steiger S, Hundemer M, Weinhold N, Rippe K, Fröhling S, Eichmüller SB, Bunse L, Müller-Tidow C, Goldschmidt H, Platten M, Raab MS, and Friedrich M (2022) IgE type multiple myeloma exhibits hypermutated phenotype and tumor reactive T cells. Journal for ImmunoTherapy for Cancer 10. Link to full text
- Kustermann M, Dasari P, Knape I, Keltsch E, Liu J, Pfluger S, Osen W, Holzmann K, Huber-Lang M, Debatin KM, and Strauss G (2022) Adoptively Transferred in vitro-Generated Myeloid-Derived Suppressor Cells Improve T-Cell Function and Antigen-Specific Immunity after Traumatic Lung Injury. J Innate Immun 1-18.
- Hernández-Malmierca P, Vonficht D, Schnell A, Uckelmann HJ, Bollhagen A, Mahmoud MAA, Landua SL, Salm EVd, Trautmann CL, Raffel S, Grünschläger F, Lutz R, Ghosh M, Renders S, Correia N, Donato E, Dixon KO, Hirche C, Andresen C, Robens C, Werner PS, Boch T, Eisel D, Osen W, Pilz F, Przybylla A, Klein C, Buchholz F, Milsom MD, Essers MAG, Eichmüller SB, Hofmann W-K, Nowak D, Hübschmann D, Hundemer M, Thiede C, Bullinger L, Müller-Tidow C, Armstrong SA, Trumpp A, Kuchroo VK, and Haas S (2022) Antigen presentation safeguards the integrity of the hematopoietic stem cell pool. Cell Stem Cell 29(5):760-75 e10 doi: 10.1016/j.stem.2022.04.007.
Kilian M, Friedrich M, Sanghvi K, Green E, Pusch S, Kawauchi D, Löwer M, Sonner JK, Krämer C, Zaman J, Jung S, Breckwoldt MO, Willimsky G, Eichmüller SB, Deimling Av, Wick W, Sahm F, Platten M, and Bunse L (2022) T cell receptor therapy targeting mutant capicua transcriptional repressor in experimental gliomas. Clin Cancer Res 28:378-389.
(*): equal contribution, members of the research group are listed in bold
Get in touch with us