Synthesis Club
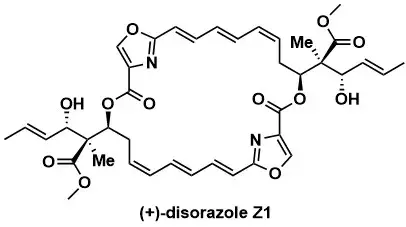
Total Synthesis of (+)-Disorazole Z1
Chem. Eur. J. 2025, 31, e202501452
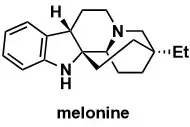
Total Synthesis of Melanine
Org. Lett. 2025, 27, 2065–2068
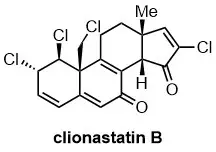
Asymmetric Total Synthesis of Clionastatins A and B
W. Ju, X. Wang, H. Tian, J. Gui J. Am. Chem. Soc. 2021, 143, 13016–13021.
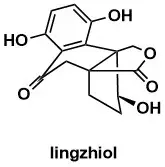
A General Entry to Ganoderma Meroterpenoids: Synthesis of Applanatumol E, H, and I, Lingzhilactone B, Meroapplanin B, and Lingzhiol
A. Rode, N. Müller, O. Kováč, K. Wurst, T. Magauer Org. Lett. 2024, 26, 9017–9021.
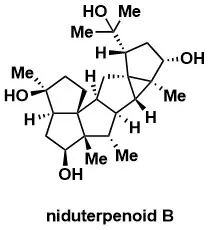
Total Synthesis of the Hexacyclic Sesterterpenoid Niduterpenoid B via Structural Reorganization Strategy
Xue, Y.; Hou, S.-H.; Zhang, X.; Zhang, F.-M.; Zhang, X.-M.; Tu, Y.-Q. J. Am. Chem. Soc. 2024, 146, 254454–25450.
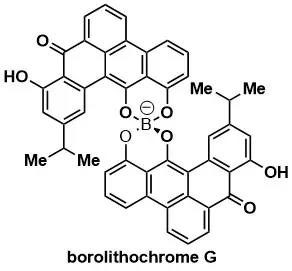
Total Syntheses of Borolithochromes A, D and G
K. Kirita, H. Matsumoto, G. Endo, S. Hosokawa Angew. Chem. Int. Ed. 2024, 63, e202400586
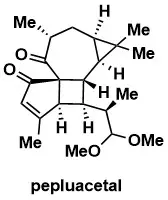
Total Synthesis of the Euphorbia Diterpenoid Pepluacetal
Liu, M.; Wu, C.; Xie, X.; Li, H.; She, X. Angew. Chem. Int. Ed. 2024, 68, e202400943.

Enantioselective Synthesis of Pactamycin, a Complex Antitumor Antibiotic
Justin T. Malinowki, Robert J. Sharpe, Jeffrey S. Johnson Science, 2013, 340, 180–182.
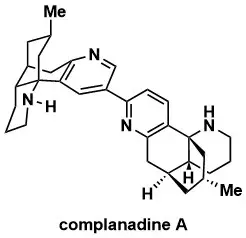
Concise Total Synthesis of Complanadine A
D. Ma, B. S. Martin, K. S. Gallagher, T. Saito, and M. Dai Synthesis 2024, 56, 107–117; J. Am. Chem. Soc. 2021, 143, 16383–16387.
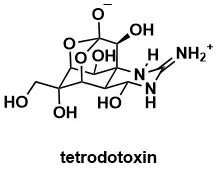
A concise synthesis of tetrodotoxin
Konrad, D.B., Rühmann, K., Ando, H., Hetzler, B.E., Strassner, N., Houk, K.N., Matsuura, B.S., Trauner, D. Science, 2022, 377, 411–415.
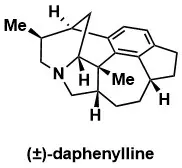
Navigating Excess Complexity: Total Synthesis of Daphenylline
Wright, BB. A., Regni, A., Chaisan, N., Sarpong, R. J. Am. Chem. Soc. 2024, 146, 1813–1818.
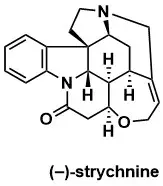
Asymmetric Total Syntheses of (–)- and (+)-Strychnine and the Wieland–Gumlich Aldehyde
Steven D. Knight, Larry E. Overman, Garry Pairaudeau J. Am. Chem. Soc. 1995, 117, 5776–5788.
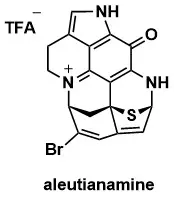
Total Synthesis of Aleutianamine
Hao Yu, Zachary P. Sercel, Samir P. Rezgui, Jonathan Farhi, Scott C. Virgil, Brian M. Stoltz J. Am. Chem. Soc. 2023, 145, 25533–25537.

Total Synthesis of Metaphanine and Oxoepistephamiersine
Ya-Kui Sun, Jin-Bao Qiao, Yu-Meng Xin, Qin Zhou, Zhi-Hua Ma, Hui Shao, Yu-Ming Zhao Angew. Chem. Int. Ed. 2023, 62, e202310917.
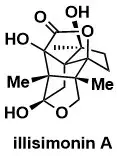
Asymmetric Total Synthesis of Illisimonin A
Etling, C., Tedesco, G., Di Marco, A., Kalesse, M. J. Am. Chem. Soc. 2023, 145, 7021–7029.
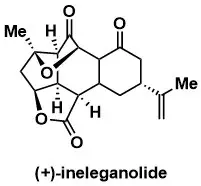
A Convergent Total Synthesis of (+)-Ineleganolide
Gross, Benjamin M.; Han, Seo-Jung; Virgil, Scott C.;Stoltz, Brian M. J. Am. Chem. Soc. 2023, 145, 7763–7767.
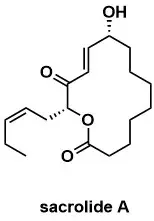
Enantioselective total synthesis of sacrolide A
T. Mohri, Y. Ogura, R. Towada, S. Kuwahara Tetrahedrom Lett. 2017, 58, 4011-4013.
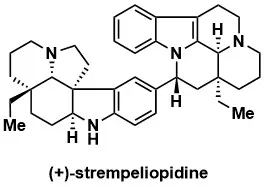
Total Synthesis of Strempeliopidine and Non-Natural Stereoisomers through a Convergent Petasis Borono–Mannich Reaction
Rand, A.W.; Gonzalez, K.J.; Reimann, C.E.; Virgil, S.C; Stoltz, B.M. J. Am. Chem. Soc. 2023, 145, 7278–7287.

Enantioselective Total Synthesis of (−)-Caulamidine A
Zhouyang Zhu and Thomas J. Maimone J. Am. Chem. Soc. 2023, 145, 14215–14220.
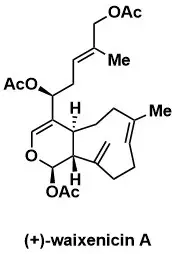
Total Syntheses of (+)-Waixenicin A, (+)-9-Deacetoxy-14,15-deepoxyxeniculin, and (−)-Xeniafaraunol A
Steinborn, C., Huber, T., Lichtenegger, J., Plangger, I., Wurst, K., & Magauer, T. J. Am. Chem. Soc. 2023, 145, 11811–11817.
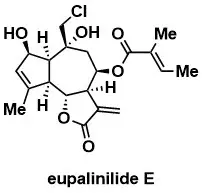
Synthesis of Eupalinilide E, a Promoter of Human Hematopoietic Stem and Progenitor Cell Expansion
Johnson, T.C., Chin, M.R., Han, T., Shen, J.P., Rana, T., Siegel, D. J. Am. Chem. Soc. 2016, 138, 6068–6073
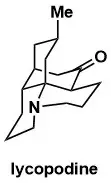
Enantioselective Total Synthesis of Lycopodine
Hua Yang, Rich G. Carter and Lev N. Zakharov J. Am. Chem. Soc. 2008, 130, 9238–9239.
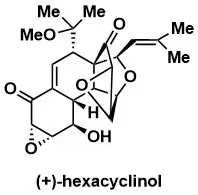
Total Synthesis and Structure Assignment of (+)-Hexacyclinol
Porco, J.A., Jr.; Su, S.; Lei, X.; Bardhan, S.; Rychnovsky, S.D. Angew. Chem. Int. Ed. 2006, 45, 5790 – 5792.

Total Synthesis of Vilmoraconitine
Ji, J; Chen, J; Qin, S; Li, W; Zhao, J; Li, G; Song, H; Liu, X-Y; Qin, Y J. Am. Chem. Soc. 2023, 145, 3903–3908.
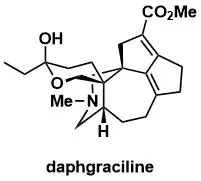
Total Synthesis of Yuzurine-type Alkaloid Daphgraciline
Li, L.-X.; Min, L.; Yao, T.-B.; Ji, S.-X.; Qiao, C.; Tian, P.-L.; Sun, J.; Li, C.-C. J. Am. Chem. Soc. 2022, 144, 18823 – 18828.
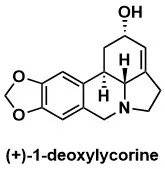
The First Asymmetric Synthesis of a Lycorine Alkaloid. Total Synthesis of (+)-1-Deoxylycorine
A. G. Schultz, M. A. Holoboski, M. S. Smyth J. Am. Chem. Soc. 1993, 115, 7904–7905.

Scalable Total Synthesis of (–)-Vinigrol
Xuerong Yu, Lianghong Xiao, Zechun Wang, Tuoping Luo J. Am. Chem. Soc. 2019, 141, 3440–13443.
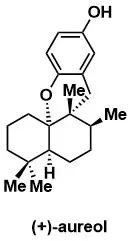
Highly Efficient Total Synthesis of the Marine Natural Products (+)-Avarone, (+)-Avarol, (−)-Neoavarone, (−)-Neoavarol and (+)-Aureol
J. Sakurai, T. Oguchi, K. Watanabe, H. Abe, S. Kanno, M. Ishikawa, and T. Katoh Chem. Eur. J. 2008, 14, 829 – 837.
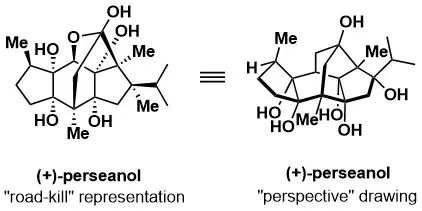
A 16-step synthesis of the isoryanodane diterpene (+)-perseanol
Han, A.; Tao, Y.; Resiman, S. E. Nature 2019, 573, 563–568.
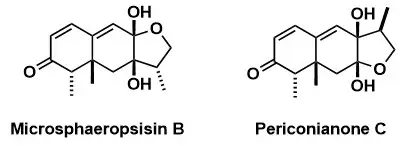
The Furan Shuffling Hypothesis: A Biogenetic Proposal for Eremophilane Sesquiterpenoids
N. Lardon, R. Liffert, A. Linden, K. Gademann Angew. Chem. Int. Ed. 2019, 58, 7004–7007.
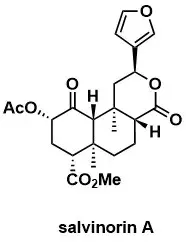
Second-generation synthesis of salvinorin A
H. Hagiwara, Y. Suka, T. Nojima, T. Hoshi, T. Suzuki Tetrahedron 2009, 65, 4820–4825.

