Division of Cellular Immunology
Prof. Dr. Hans-Reimer Rodewald
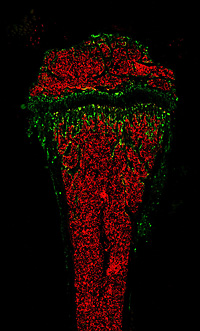
Immunfluorescence staining of lymphocytes (red) and osteoclasts (green) in bone marrow of a mouse femur.
© dkfz.de
The Division of Cellular Immunology investigates cell and organ development in the immune system as well as their immunological functions.
Hematopoietic stem cells. Hematopoietic stem cells (HSC) have been widely studied by transplantation into immune- and blood-cell-depleted recipients. To address HSC functions under physiological, non-transplant conditions, we generated a mouse model enabling inducible genetic labelling of HSC at different time points of embryonic development and in the adult bone marrow. Our data reveal fundamental differences between normal maintenance of the hematopoietic system and its re-establishment after transplantation. Measurements of hematopoietic flow emerging from HSC in situ, together with mathematical modelling (collaboration with the group of Thomas Höfer, Theoretical Systems Biology, DKFZ), now provide a quantitative framework for studying hematopoiesis in health and disease in situ (Busch et al. Nature 2015).
Barcoding. To reveal the fates emerging from HSC in vivo, we developed an artificial DNA recombination locus (termed Polylox) that enables broadly applicable endogenous barcoding based on the Cre–loxP recombination system. Polylox recombination in situ reaches a practical diversity of several hundred thousand barcodes, allowing tagging of single cells. Combining of the Polylox system with our HSC fate mapping (see above) uncovered HSC fates realized in vivo on a clonal level. Together with the group of Thomas Höfer we found that the adult HSC compartment is a mosaic of embryo-derived HSC clones, some of which are unexpectedly large. The spreading of barcodes, both after induction in embryos and in adult mice, revealed a basic split between common myeloid–erythroid development and common lymphocyte development (Pei, Feyerabend et al. Nature 2017), supporting the long-held but contested view of a tree-like hematopoietic structure.
Tissue resident macrophages. While HSC are responsible for the generation of most hematopoietic lineages, tissue macrophages represent a notable exception. By applying non-myeloablative transplantation of HSC and fate mapping, we showed that the vast majority of adult tissue macrophages in liver, brain, epidermis and lung arise in development from the yolk sac and are sustained in adult life without input from HSC-derived progenitors (Gomez Perdiguero, Klapproth et al. Nature 2015).
T cell development and T cell leukaemia. T cells develop exclusively in the thymus, a primary immunological organ. We discovered that thymocytes undergo transformation if they non-physiologically persist in the thymus. This occurs with surprisingly high incidence if the influx of fresh progenitors into the thymus is interrupted. We found that in mice T cell progenitor turnover in the thymus is regulated by cell competition between ‘young’ bone-marrow-derived and ‘old’ thymus-resident thymocytes. Disruption of this competition results in T-cell acute lymphoblastic leukaemia (T-ALL), resembling the human disease in pathology, genomic lesions, leukaemia-associated transcripts, and in activating mutations in Notch1 (Martins et al. Nature 2014).
Mast cells. Mast cells play key roles in allergic diseases, yet their ‘true’ immunological functions remain poorly understood. We have generated a mouse mutant that is completely mast cell deficient but has a normal immune system, thus representing an excellent model to clarify physiological mast cell functions beyond allergy. Our results using this mouse have called for a systematic re-evaluation of described immunological functions described for mast cells (for example Gutierrez et al. Cell Metab. 2015).
Future Outlook
We study dynamic processes of cell differentiation from stem and progenitors under steady state as well as under challenge conditions. We investigate functions of tissue resident macrophages. We apply our DNA barcoding system (Polylox) to unravel stem cell fates, lineage relationships and clone sizes. We aim at a deeper insight into the early cellular and molecular events underlying the origin of T-ALL. We work on mast cell physiology.

